To the Editor: Risperidone and clozapine are known to potentially cause drug-induced hepatitis in 0.01%–0.1% of patients (
1–
3). Paliperidone, which is pharmacologically identical to 9-hydroxyrisperidone, exhibits high affinities for dopamine type 2 (D
2) and serotonin 5-HT
2 receptors but does not undergo significant hepatic metabolism (
4). The drug is well tolerated in patients with poor hepatic function and seems unlikely to be susceptible to metabolic drug interactions. We report a case of risperidone-induced hepatitis, which remitted after switching from treatment with risperidone to treatment with paliperidone, in a patient receiving dual antipsychotics.
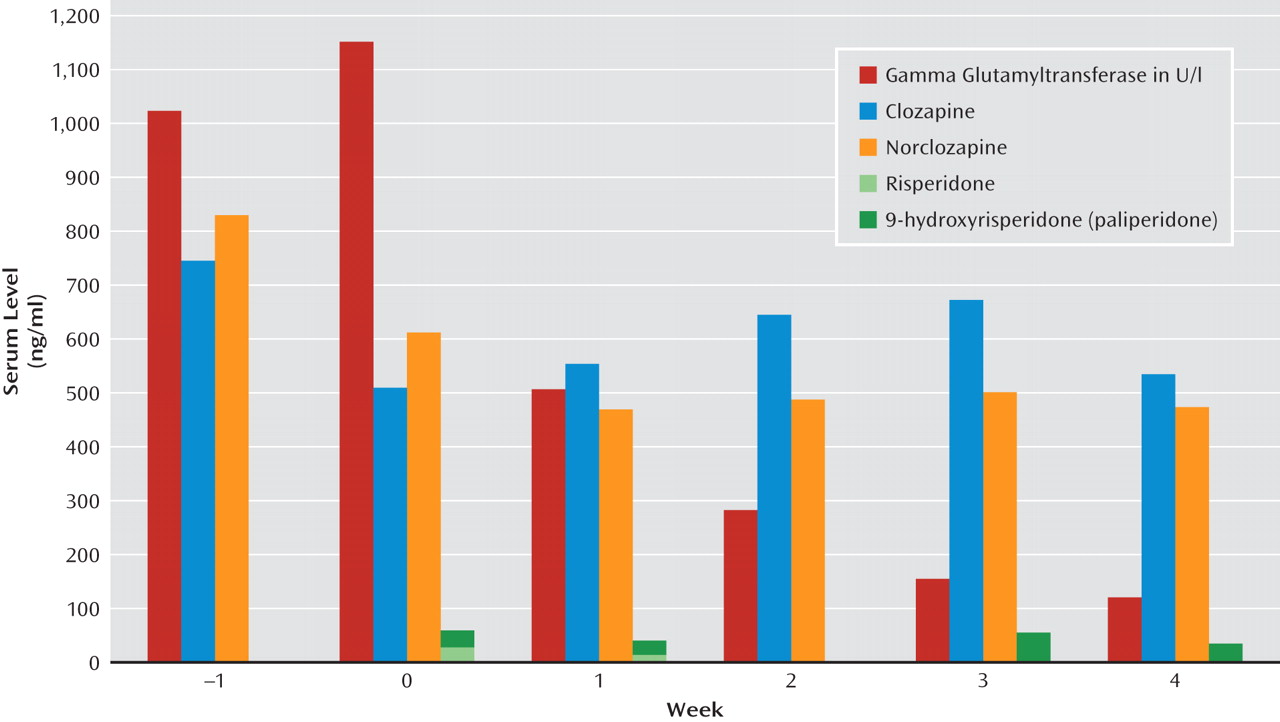
"Mrs. J" was a 43-year-old female patient suffering from schizophrenia. She was being treated with risperidone, 6 mg per day, and clozapine, 600 mg per day, when she was admitted to the Department of Internal Medicine because of drug-induced hepatitis. Clinical and laboratory tests revealed an icterus; beer-brown urine; and highly elevated liver enzymes, with a maximum of 1.159 U/l for gamma glutamyltransferase (normal range: 6–42 U/l), 206 U/l for alkaline phosphatase (normal range: 10–35 U/l), and 75 U/l for alanine transaminase (normal range: 10–35 U/l). Serum levels for risperidone active moiety were within the therapeutic range of 20–60 ng/ml (risperidone: 26 ng/ml; 9-hydroxyrisperidone: 30 ng/ml). Clozapine serum levels were also within the therapeutic range (508 ng/ml; reference range: 350–600 mg/ml), with 609 ng/ml for norclozapine (metabolite).
Risperidone treatment was discontinued, and paliperidone, 9 mg per day, was started. Clozapine doses were slightly reduced to 575 mg in the third week as a result of increasing plasma levels. The patient's psychopathology remained stable throughout clinical treatment. Liver enzymes as well as serum levels for paliperidone and clozapine were measured weekly. Aspartate transaminase levels returned to normal within 1 week, and alanine transaminase levels returned to normal after 16 days. After 4 weeks, gamma glutamyltransferase levels reached the lowest measured value, at 112 U/l, representing a 90% reduction of the initial value. Serum levels at that time were 34 ng/ml for paliperidone, 536 ng/ml for clozapine, and 483 ng/ml for norclozapine. Analyses of clozapine and norclozapine serum levels revealed that in a state of high levels of hepatotoxicity, serum levels for the metabolite were considerably higher than those for the parent drug, whereas the ratio of serum levels between the parent drug and its metabolite was higher for the parent drug after liver enzymes approximated normal ranges.
Our case shows that, first, hepatitis that was most likely induced by risperidone completely remitted after switching to paliperidone. Since treatment with clozapine remained almost unchanged, it seems plausible that risperidone rather than clozapine induced hepatotoxicity, not disregarding that clozapine itself or the combination of both drugs could have been the cause of the hepatitis. Risperidone is metabolized to 9-hydroxyrisperidone via cytochrome P450 (CYP) 2D6, while paliperidone does not undergo significant hepatic metabolism. Therefore, it can be recommended that in cases of hepatotoxicity, paliperidone is a well-tolerated alternative to treatment with risperidone.
Second, declining signs of hepatotoxicity led to an inversion of the clozapine/norclozapine ratio. As seen in
Figure 1, serum levels for the metabolite were initially higher than those for the parent drug (ratio <1), but the reverse was observed with normalization of liver function (ratio >1). A possible explanation may be that in the state of acute inflammation, different metabolizing enzymatic pathways are activated on the one hand, while other metabolic pathways are likely working insufficiently as a result of the acute hepatitis. Consequently, there is a shift among the levels of the different metabolites of clozapine (e.g., norclozapine, clozapine-n-oxide) in acute hepatitis, and the ratio returns to its "normal value" after normalization of liver function.