Tourette's syndrome is a neuropsychiatric disorder that preferentially affects the face, neck, shoulders, and vocal apparatus to produce involuntary motor and vocal tic behaviors. Anatomical, functional, and lesion studies suggest that Tourette's syndrome is caused by a failure of cortico-striato-thalamo-cortical circuits to inhibit the somatosensory urges and associated motor enactments that constitute tic behaviors (
1–
8). However, this hypothesis has yet to be verified by studies of isolated neural activity of the functionally distinct regions that compose cortico-striato-thalamo-cortical circuits, in tics that occur spontaneously in Tourette's syndrome patients compared with healthy individuals performing similar behaviors voluntarily.
Prior functional imaging studies of Tourette's syndrome have attempted to correlate regional brain activity with the temporal occurrence of tics (
9,
10), but they have not compared activity during spontaneous tics in Tourette's syndrome patients with activity during similar voluntary behaviors in healthy subjects. They also have not assessed causality among brain regions during tics because they could not detect isolated neural activity from each of the functionally distinct regions that compose cortico-striato-thalamo-cortical circuits. One study computed the cross-correlations of brain activity in the primary motor cortex with activity in the somatosensory and supplementary motor cortices and subcortical nuclei and then compared those correlations between Tourette's syndrome patients during tic behaviors and healthy subjects simulating tic behaviors (
11). Group differences were detected only in the supplementary motor area. This limited finding might be attributed to the inherent constraints of region-of-interest analyses, which use a predetermined set of regions rather than those in which activations are found empirically (
12).
Despite these strong a priori hypotheses, sensorimotor and control portions of cortico-striato-thalamo-cortical circuits involve multiple brain regions, and the precise location of the regions that generate, control, or modulate tic behaviors and premonitory urges in persons with Tourette's syndrome remains uncertain. We therefore used a data-driven approach—independent component analysis—to detect coherent, spatially localized, and task-related blood-oxygen-level-dependent (BOLD) activity in each run type of fMRI data. Independent component analysis is a multivariate method for analysis of fMRI data sets that allows for the separation of noise from physiological signals without knowledge a priori of the defining characteristics of the noise and signal or how they are intermixed (
14,
15). Independent component analysis maximizes mutual information within a component while minimizing it between components to identify BOLD activity within brain regions that are functionally distinct and spatially isolated from one another (
14,
16,
17). Because BOLD activity is an index of task-related neural activity (
18), the regional isolation of BOLD activity using independent component analysis is thought to identify regions in which neural activity is coherent (i.e., where BOLD signals are mutually phase-coupled) and that therefore constitute either a neural circuit or a portion of one circuit (
14,
16,
17). We applied independent component analysis, together with a hierarchical extension of our partner matching algorithm (
17), to identify independent components that were reproducible across individuals and across each fMRI run type during the production of tics or tic-like behaviors. To clarify how circuits that generate tics interact with those that control them, we also calculated the Granger causality index (
19,
20) as a measure of causal interactions among components of cortico-striato-thalamo-cortical circuits that are known to generate or control motor behaviors (
5,
21–
24).
Discussion
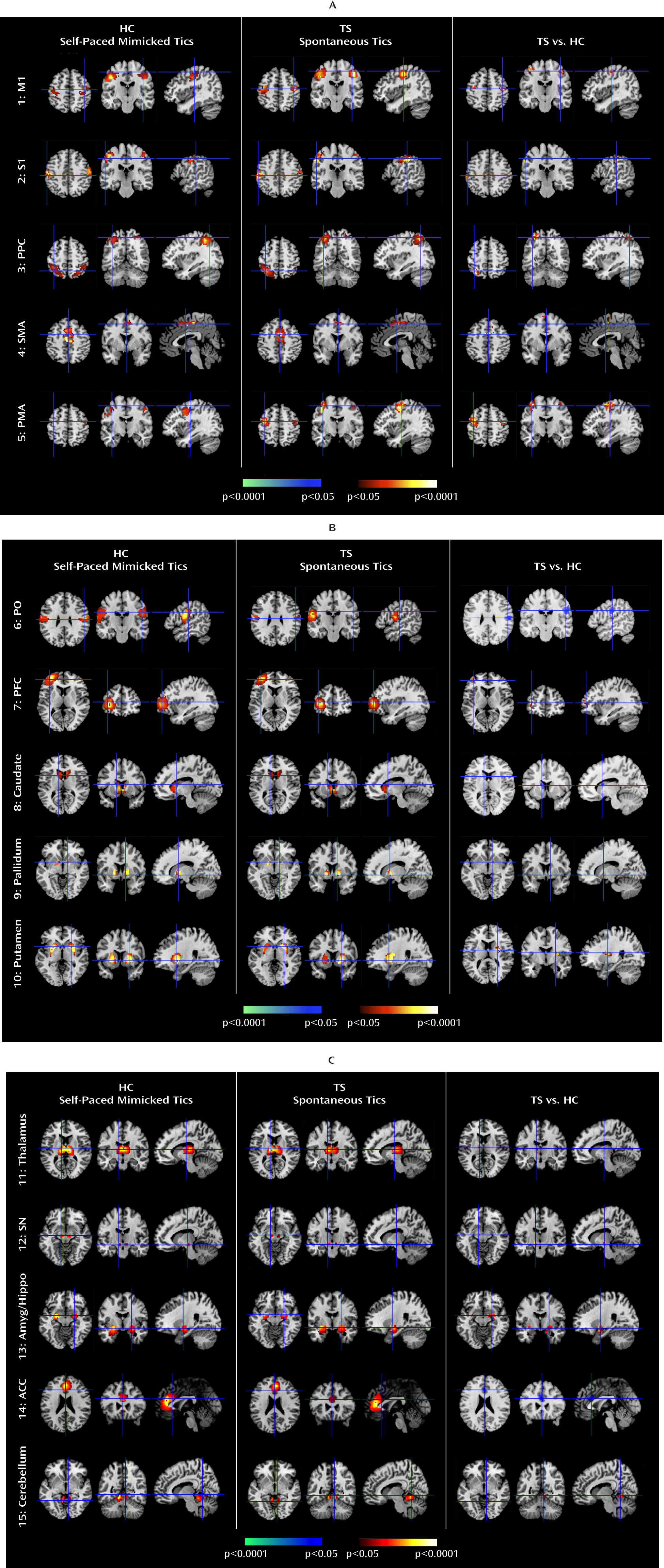
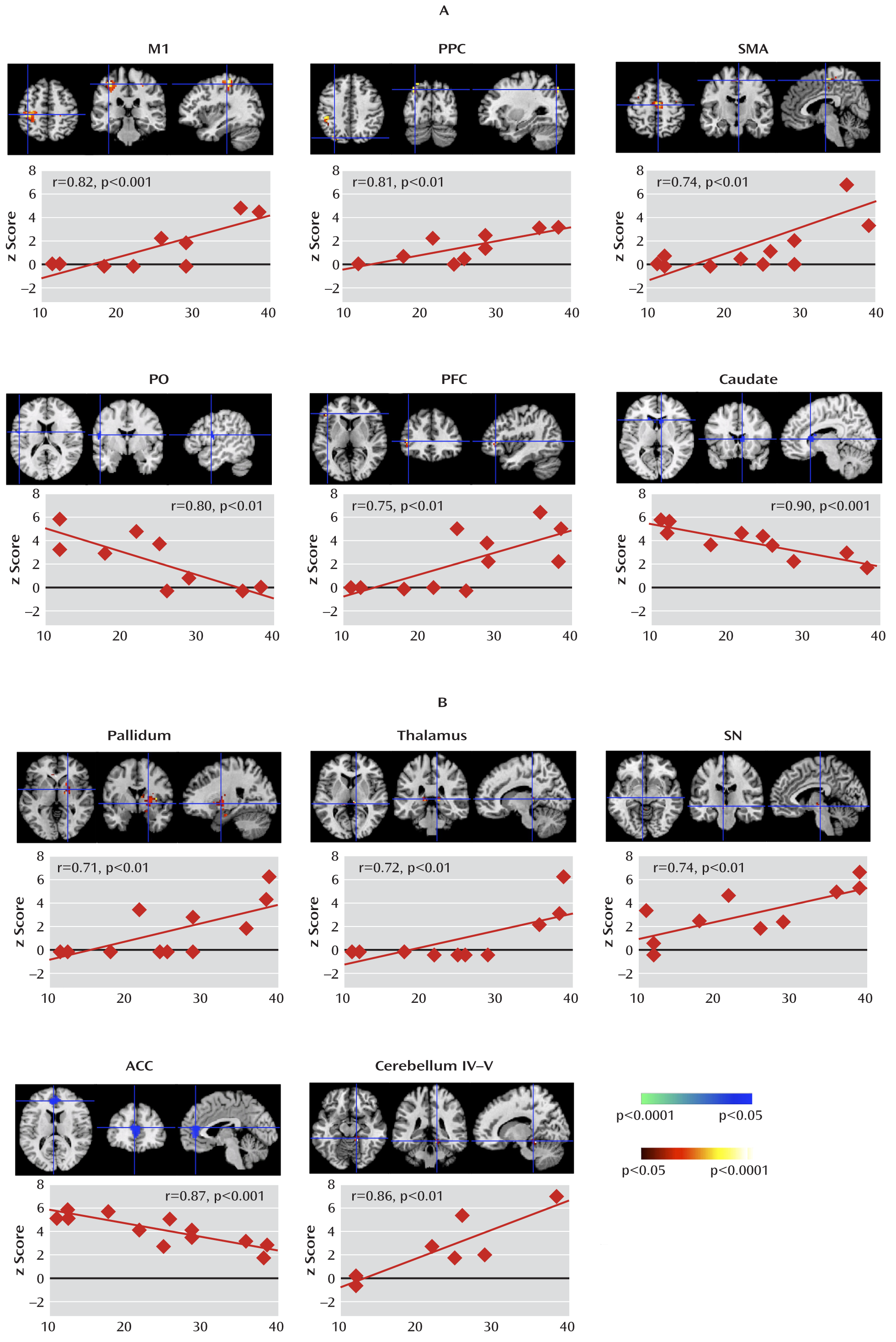
We isolated neural activity within independent components representing portions of cortico-striato-thalamo-cortical circuits that are involved in planning, controlling, and executing motor behaviors, including the primary motor, supplementary motor, premotor, somatosensory, and prefrontal cortices and the putamen, caudate, pallidum, thalamus, and substantia nigra (
21,
24,
29). We detected these components during the expression of spontaneous tics in the Tourette's syndrome group and during the voluntary imitation of a facial tic in both the Tourette's syndrome and healthy comparison groups. Activity in the Tourette's syndrome group was greater during spontaneous tics than in the comparison group during self-paced mimicked tics in most sensorimotor portions of cortico-striato-thalamo-cortical loops. Moreover, activity within these regions correlated positively with the severity of tics in the Tourette's syndrome group, indicating that increasing activity within sensorimotor portions of these loops accompanied more severe tic behaviors. These findings demonstrate that tics engage the same neural circuits that support the expression of normal voluntary motor behaviors in healthy individuals. More severe tic symptoms simply produce more activity in these motor circuits.
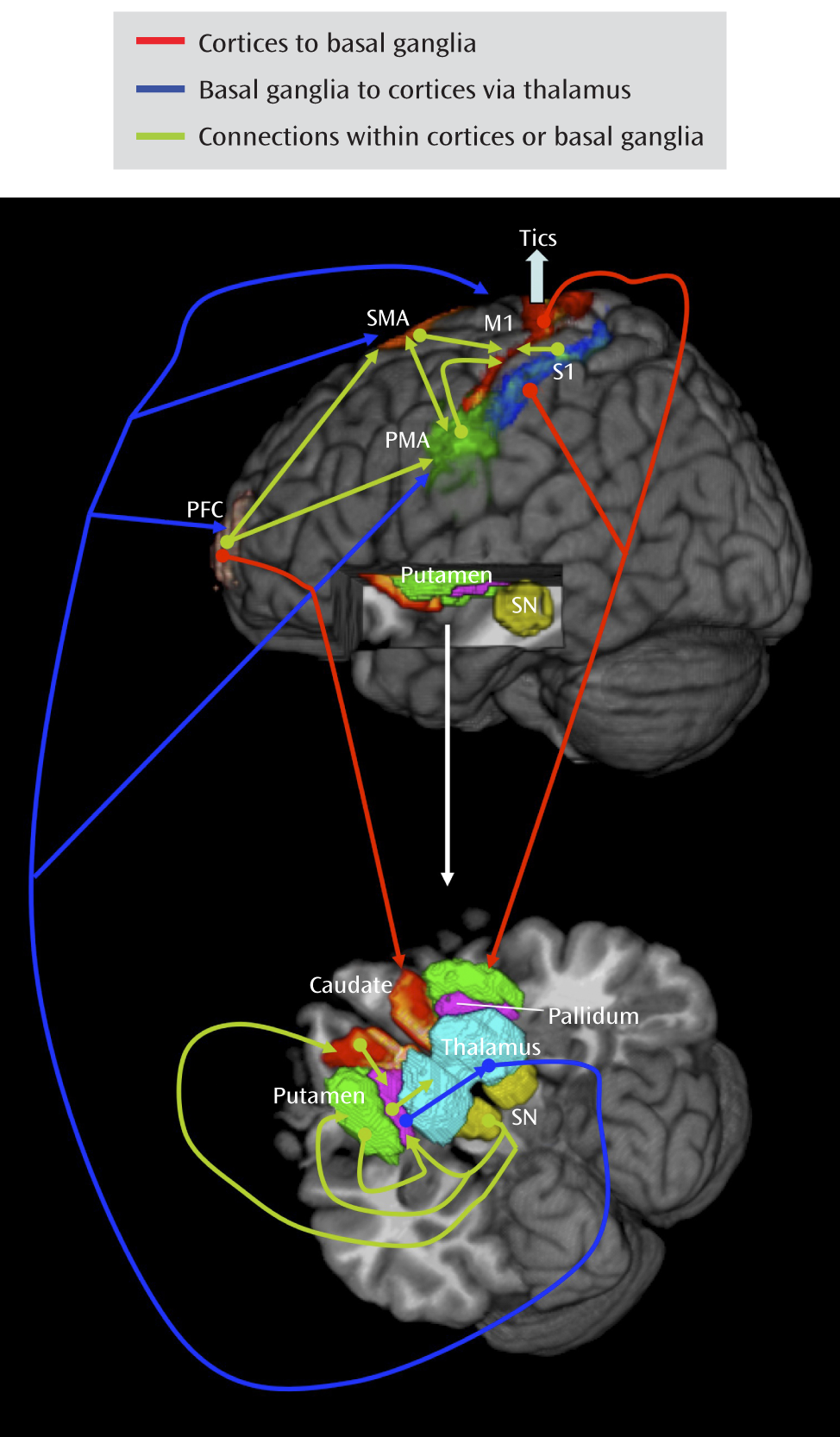
Analyses of Granger causality demonstrated that the activity within sensorimotor pathways in the Tourette's syndrome group during the spontaneous generation of tics follows a chain of causal influences within the pathways that have long been postulated in the generation of movement: from the premotor to supplementary motor area, from the primary somatosensory cortex to the primary motor cortex, from the pallidum via the thalamus to the supplementary motor area, and from the substantia nigra to the striatum. These measures of causal influence were stronger in the Tourette's syndrome group than in the comparison group, suggesting an enhanced functional coupling of activity between successive nodes in this circuit in Tourette's syndrome. Furthermore, the top-down causal influence from the primary motor cortex to the putamen during spontaneous tics, and the bottom-up influence from the pallidum to the primary motor cortex via the thalamus during voluntary tics, correlated positively with tic severity, confirming that a stronger generation of tics may be caused by a greater interaction between the motor cortices and striatum.
In contrast, activity in the anterior cingulate, parietal operculum, and caudate was significantly less in the Tourette's syndrome group during spontaneous tics than in the comparison group during voluntary mimicking tics, with less activity in each of these regions in the Tourette's syndrome group accompanying more severe symptoms. The anterior cingulate and caudate represent cognitive control portions of cortico-striato-thalamo-cortical loops (
23,
30) that can modulate activity in sensorimotor portions of the striatum via striato-nigro-striatal (
31) and striato-thalamo-striatal circuits (
32). Activity in the anterior cingulate and caudate are known to increase during the successful and willful suppression of tic behaviors (
33), with greater caudate activation accompanying fewer tic symptoms. Thus, reduced activity in the anterior cingulate and caudate during spontaneous tics in the Tourette's syndrome group likely represented deficient engagement of circuits that inhibit either tic behaviors or the sensorimotor urges that produce them. These findings remained significant when comparing activity during spontaneous tics in the Tourette's syndrome group with activity during cue-paced mimicked tics in the comparison group, demonstrating that group differences were not simply the consequence of spontaneous tics being generated by cues (the premonitory urge). Thus, our findings suggest that tics are caused by the combined effects of excessive activity in motor pathways and reduced activity in control portions of cortico-striato-thalamo-cortical circuits, consistent with evidence of abnormal development of control regions in Tourette's syndrome patients (
34).
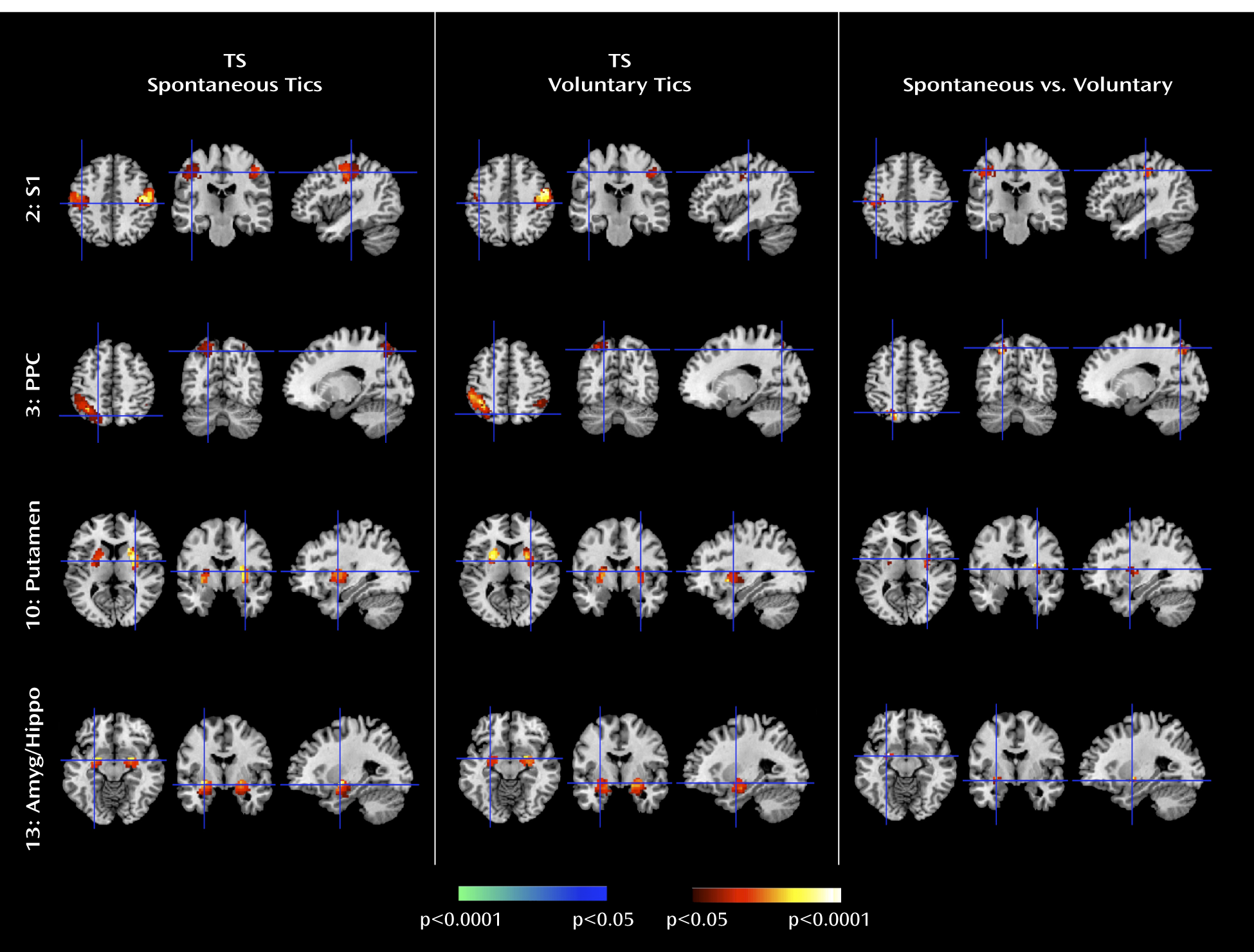
The Tourette's syndrome group exhibited greater activity in the putamen, somatosensory cortices (including the primary somatosensory cortex and parietal operculum), and amygdala/hippocampus complex during the spontaneous generation of tics than during the voluntary imitation of tics, a contrast designed to help identify regions that contribute to the generation of spontaneous tics. Conceptually, spontaneous tics should differ from voluntary tics in the presence of 1) a “tic generator” during spontaneous tics, 2) premonitory urges associated with spontaneous tics (with the urges themselves possibly being the tic generators), or 3) volitional control during voluntary tics. Differences in neural activity between spontaneous and voluntary tics could derive from any of these three sources. However, given the intense sensory and emotional salience of the premonitory urge in the subjective experience of Tourette's syndrome patients (
35,
36), the differences in activity that we detected in primarily sensory and emotional pathways seem most likely to derive from intense sensory and emotional experiences associated with premonitory urges in the Tourette's syndrome group. Therefore, we suspect that greater activity in somatosensory cortices likely represented the sensory features of those urges, whereas greater activity in the amygdala/hippocampus complex likely represented either the emotional discomfort associated with the urges before the tic or the relief experienced following the tic. The stronger Granger causality index from the primary somatosensory cortex to the primary motor cortex during spontaneous tics in the Tourette's syndrome group than during self-paced mimicked tics in the comparison group further suggests that activity in sensory cortices from these premonitory urges causally influenced activity in motor pathways.
Greater activity found in the amygdala in the Tourette's syndrome group can have alternative explanations. The emotional experiences signaled by the amygdala can influence motor pathways via projections to the ventral striatum, which in turn influences motor areas in the dorsal striatum (
31). These pathways are similar to those implicated in addictive behaviors (
37), which, like the tics in Tourette's syndrome, have compulsory qualities.
The Tourette's syndrome group had greater neural activity than the comparison group within the substantia nigra during the performance of spontaneous and voluntary tics. Causal influences of the substantia nigra on the caudate were also stronger in the Tourette's syndrome group during spontaneous tics than in the comparison group during the performance of both self-paced and cue-paced mimicked tics. The pars compacta of the substantia nigra contains dopaminergic neurons that project to the striatum, and excessive striatal dopaminergic activity has long been suspected to play a role in Tourette's syndrome (
38). Our findings may therefore reflect overactive nigrostriatal dopaminergic activity in the disorder.
Our findings permit a detailed understanding of the circuit-based disturbances that together generate tics. They suggest that increased activity in the primary somatosensory cortex, putamen, and amygdala/hippocampus may represent activity associated with the premonitory urge and act as a trigger for tic behaviors. They also indicate that the primary sensory cortex exerts a causal influence on the putamen that is greater in the Tourette's syndrome patients than in the healthy subjects, presumably within the projection from the sensory cortex to the putamen that is known to be glutamatergic and excitatory. These findings further suggest that increased activity in the putamen in the Tourette's syndrome group exerts an increased causal influence on the pallidum, a projection that is inhibitory (γ-aminobutyric acid-ergic). The pallidum sends an inhibitory projection to the thalamus, and the thalamus in turn sends an excitatory projection to the motor cortex, a pathway that our data indicate overall had a stronger causal influence in the Tourette's syndrome group than in the comparison group. The stronger inhibitory influence of the putamen on the pallidum would therefore ultimately disinhibit thalamic excitation of the cortex, which should increase the production of tics, consistent with dysfunction in the direct pathway of cortico-striato-thalamo-cortical circuits in Tourette's syndrome (
5). Finally, our findings indicate that the putative trigger from the premonitory sensory urge and the excess activity in motor portions of cortico-striato-thalamo-cortical circuits combines with reduced activity in the anterior cingulate and caudate—the putative control portions of these circuits—to yield disinhibited and poorly regulated motor activity in proportion to tic severity.
This study has several limitations, including a small sample size, inclusion of medicated patients and patients with comorbid illnesses, and absence of adults with remitted symptoms. A follow-up study with a larger sample would help to assess more rigorously any possible effects of medications and comorbid illnesses. Our study, similar to prior studies, was unable to control stringently for the strength, duration, or pacing of tics across conditions or groups (although visually the tic behaviors were indistinguishable across conditions and groups). Finally, our partner matching technique detects independent components that are reproducible and can be compared across conditions and groups; it does not permit identification of components unique to a condition or group.
This is the first time, to our knowledge, that neural activity in all major components of a cortico-striato-thalamo-cortical circuit has been detected using an analysis of BOLD signals. Comparing activity in this circuit during a pathological involuntary behavior (spontaneous tics) with activity during a similar normal voluntary behavior (voluntary or mimicked tics) across patient and healthy comparison groups allowed us to demonstrate that tics engage the same motor circuit as normal voluntary behaviors. This comparison also permitted us to identify the somatosensory cortices, putamen, and amygdala/hippocampal complex as regions that likely subserve the experience of premonitory sensory urges and their associated emotional content. Finally, the comparison also allowed us to show that tics likely arise as a combined consequence of this sensory trigger, its contributions to the excessive activity in motor pathways, and faulty regulation from the anterior cingulate cortex and caudate nucleus.