One of the best known demonstrations of gene-by-environment effects involves a functional promoter polymorphism (5-HTTLPR) in the serotonin transporter gene (
SLC6A4) in which the short (S) allele interacts with exposure to stressful life events to predict risk for mood-related psychopathology (
6,
7). This specific gene-by-environment interaction effect on risk for psychopathology has been widely replicated in human studies of both depression and anxiety (
8,
9) and supported by nonhuman primate and rodent models (
10–
12), although there have been some unsuccessful attempts at replication (
13). One recent large-scale meta-analysis provided strong support for this gene-by-environment interaction effect (
7).
Imaging genetics studies, which leverage functional neuroimaging to uncover the neurobiological correlates of specific genetic variants (
14), have shown a robust association between the S allele and increased amygdala reactivity during implicit processing of picture stimuli containing negative facial expressions (
15) but no changes in response to positive facial expressions (
16,
17). While these studies clearly implicate neurobiological pathways involved in mediating sensitivity to environmental stress, the specific neural mechanisms through which this gene-by-environment interaction confers risk remain largely unknown. Indeed, few studies to date have directly examined the modulatory effects of the 5-HTTLPR polymorphism on corticolimbic circuit responses during exposure to acute stress (
18,
19).
In this study, we administered an acute laboratory stressor to healthy adult women during blood-oxygen-level-dependent (BOLD) functional magnetic resonance imaging (fMRI). Focusing on a healthy population allowed us to investigate the mechanism of risk without the confounding variable of current or past psychopathology, which makes it difficult to infer cause from consequence. In this paradigm, electric shocks of uncertain intensity were threatened and unpredictably delivered to the wrist after a long anticipatory cue period of unpredictable duration, allowing for robust responses to be generated (
21). We obtained ratings of global anxiety experience and anxiety regulation success at the end of the task. Two hypotheses were examined. First, based on existing epidemiological and neuroimaging research (
8), we hypothesized that there would be an interaction between the 5-HTTLPR genotype and stress, such that in these healthy women, the short allele would be associated with greater amygdala reactivity as well as greater activation in the medial prefrontal cortex during stress exposure (
22). Second, based on the brain regions identified in these analyses as well as extensive literature on neural responses to threat (
23–
25), we hypothesized that genotype would moderate the relationship between corticolimbic BOLD responses and reports of anxiety and regulation success. Our specific focus was brain regions implicated in triggering central and peripheral responses to threat (amygdala) (
23), interoceptive processing of threat (insula) (
24), and cognitive appraisal of threat (medial prefrontal cortex) (
25), and we hypothesized that S-allele carriers would have greater brain-behavior coupling with anxiety experience but lesser coupling with regulation success.
Discussion
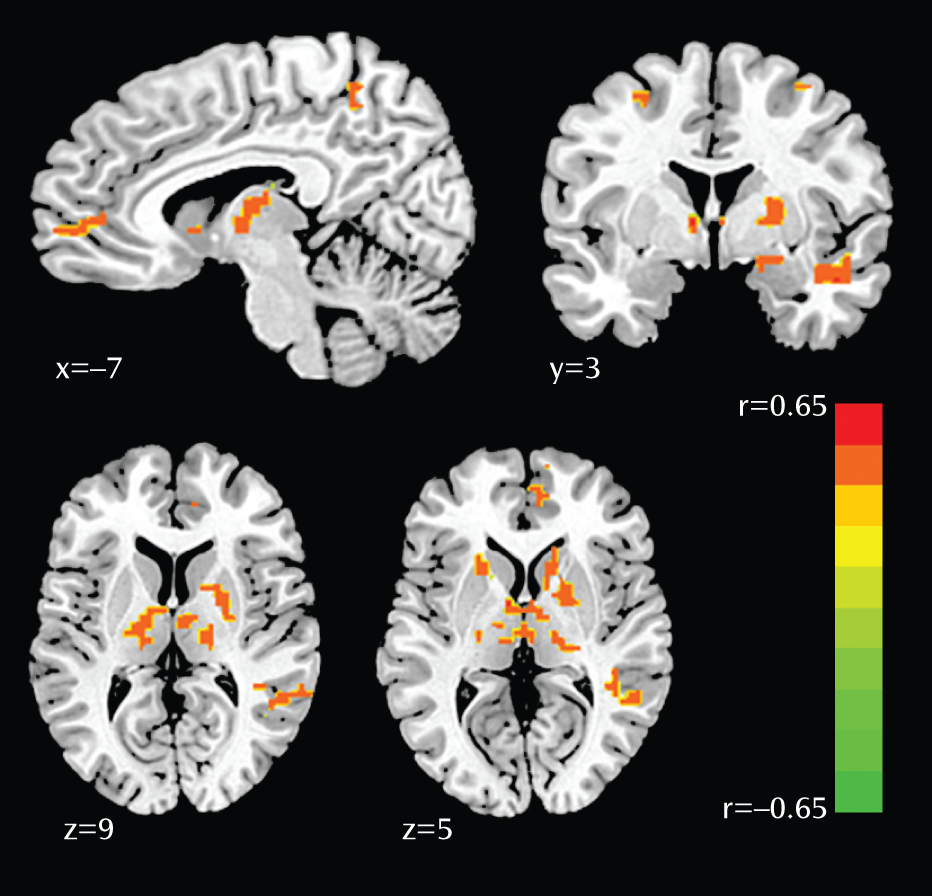
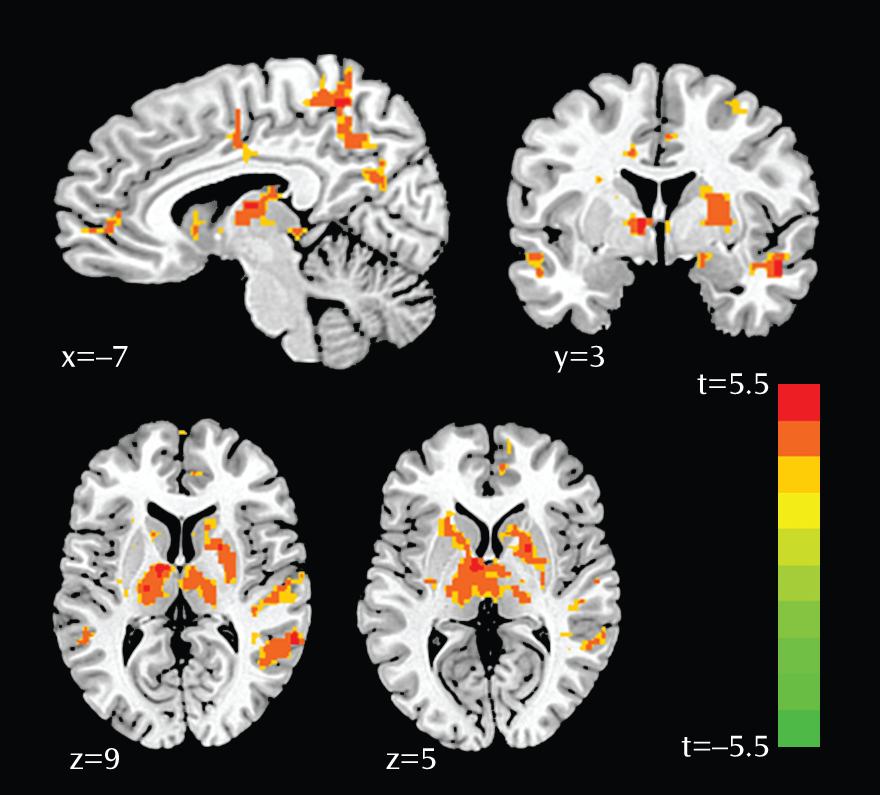
In this study, we examined neural correlates of genetic sensitivity to acute stress exposure conferred by the 5-HTTLPR short allele. Findings revealed that stress-induced activation was enhanced in the amygdala, hippocampus, anterior insula, thalamus, pulvinar, caudate, precuneus, anterior cingulate cortex, and medial prefrontal cortex in women with the homozygous SS genotype, compared with women carrying the L allele. Notably, enhanced right amygdala reactivity observed in women with the SS genotype was located in the dorsal region encompassing the central nucleus, which is known to drive behavioral and physiological arousal (
42) through interaction with the thalamus and cortex. This study also demonstrated substantial modulation of the thalamus by 5-HTTLPR genotype, particularly the dorsomedial nucleus and pulvinar. These regions are known to modulate emotion and mood; the thalamus gates sensory information to the amygdala and mediates the flow of information within the limbic system (
43). Moreover, the pulvinar is anatomically connected to both the anterior cingulate cortex and the amygdala (
44,
45) and relays emotionally salient information about the environment to the limbic system (
46). Our findings are in line with previous research indicating that the thalamus contains one of the highest levels of serotonin transporters in the brain (
47), and the pulvinar, specifically, is enlarged in S-allele carriers (
48,
49). Enhanced activation in the anterior insula, a region implicated in interoceptive processing, awareness of negative emotions, and anticipation of pain (
31,
50), provides further evidence of upregulated affective salience in individuals with the homozygous SS genotype. In addition to bottom-up affective processing, it is possible that top-down attentional mechanisms also drive sensitivity to threat. In individuals with the SS genotype, increased activation observed in the dorsal anterior cingulate and the precuneus bilaterally is consistent with biased attention toward the danger of the upcoming shock and perhaps greater responsivity in the face of uncertainty (
31). Lastly, activation in the medial prefrontal cortex has been consistently shown to be necessary for the generation of conscious appraisal of threat, and the increased activation we observed in individuals with the SS genotype may reflect altered cognitive interpretation of the shock trials.
It is striking that when a task more potent than simple images is utilized, as with previous imaging genetics studies, genetic effects in a larger and more sophisticated network for processing environmental threat are unmasked. It is possible that neural alterations associated with the SS genotype result in upregulation of affective information entering the limbic system, via the thalamus and amygdala, which drives further salience and processing in a distributed cortical network. Taken together, the present findings suggest that when individuals carrying two S alleles are exposed to acute stress, neural systems that enhance fear and arousal, modulate attention toward threat, and perseverate on emotional salience of the threat are engaged. In turn, this may be a mechanism underlying risk for psychopathology conferred by the S allele upon exposure to life stressors and may specifically speak to risk for anxiety disorders, which are characterized by chronic worry and anxiety about future events.
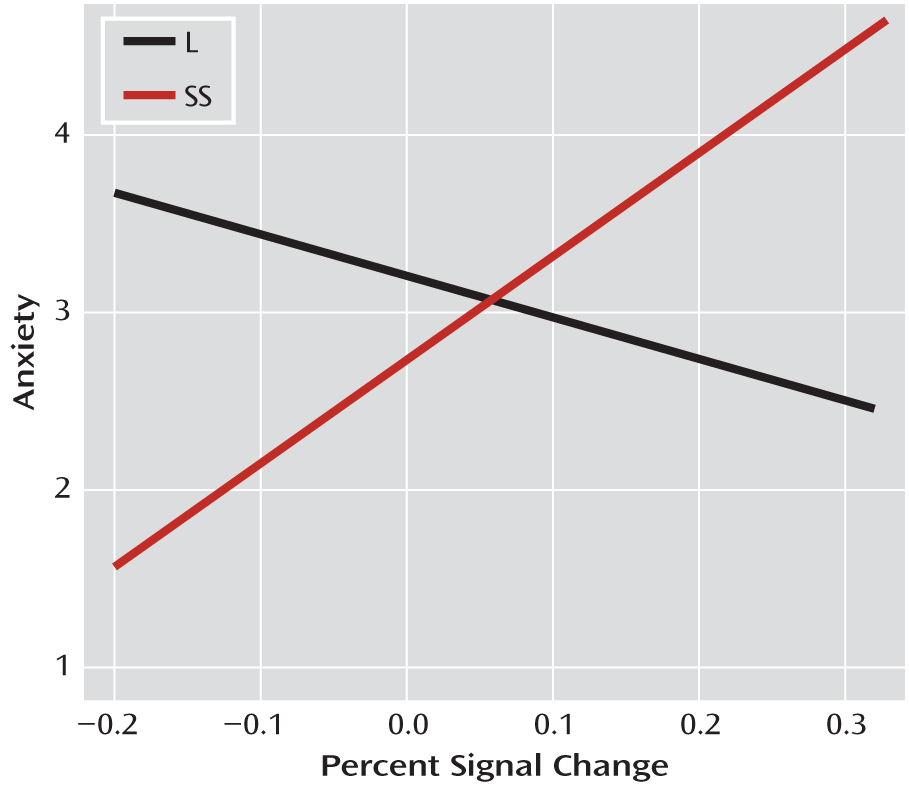
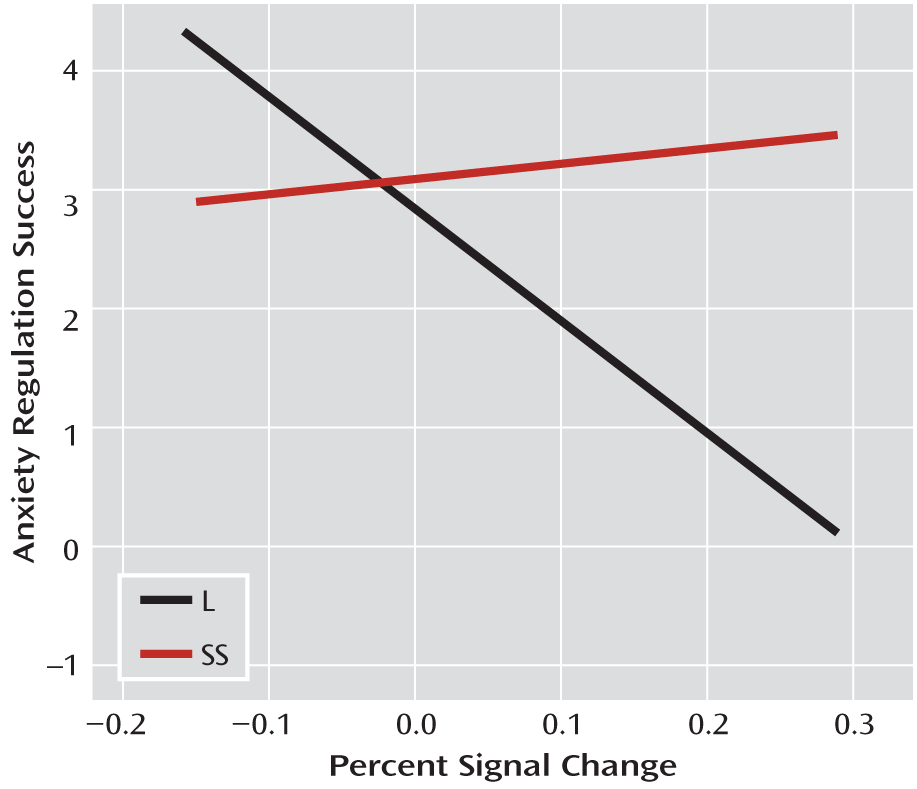
Using a task that generates visceral emotional responses allowed us to examine the relationship between psychological responses to stress, neural activity, and genotype. Results indicated a markedly increased positive relationship between medial prefrontal cortex activation and anxiety reactivity in response to the task in women with the homozygous SS genotype. This interaction effect demonstrates that medial prefrontal cortex activation signals or triggers feelings of anxiety in individuals with the SS genotype, suggesting that they may elaborate on the nature of the threat via neural circuits different from those engaged in L-allele carriers. We also found that in L-allele carriers, there was a significant negative relationship between insula activation and regulation success in response to the task. In L-allele carriers, perhaps efforts to regulate anxiety result in an extinguished response in the insula, which is a putatively more adaptive response. Interestingly, we did not find an association between 5-HTTLPR genotype and the coupling of amygdala with anxiety or regulation success. One possible interpretation is that the amygdala functions to rapidly and often unconsciously alert higher brain systems to environmental threat, but those higher structures are required to elaborate on the threat in order to generate subjective experience of anxiety and successful regulation of that anxiety. Thus, the interactions observed appear to indicate that in those with the SS genotype, compared with L-allele carriers, there are different circuit dynamics that translate differentially into behavior based on genotype. Notably, we found no direct associations between 5-HTTLPR genotype and behavioral phenotypes. This is a common occurrence when working with relatively small samples, possibly reflecting the minimal effect that genotype has on any distal behavioral phenotype (
14). These results help us to appreciate the relevance of a network of structures beyond the amygdala demonstrating effects of 5-HTTLPR in the face of stress exposure.
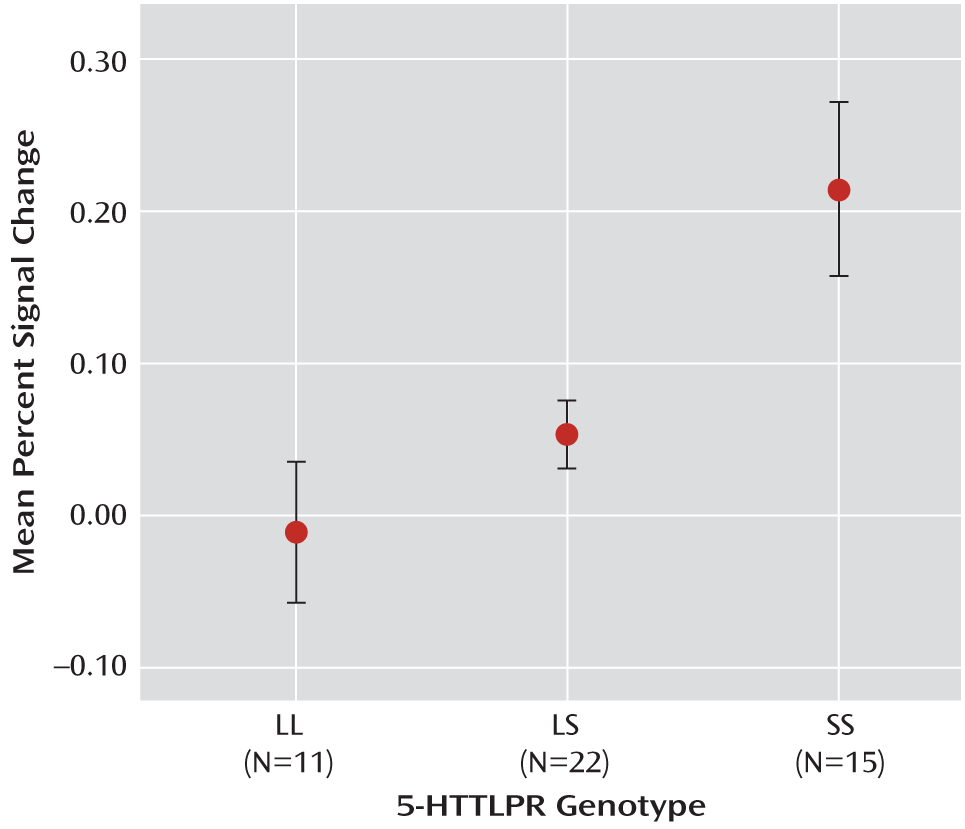
It is noteworthy that the post hoc analyses in this study clearly indicated that it was most appropriate to group individuals carrying the L allele. Consistent with previous imaging genetics studies, our a priori model was one of codominance, in which the S allele added a dose effect, and thus we conducted a whole-brain regression. However, a regression analysis is agnostic to the effect of the intermediate group, prompting us to extract the data from all of the significant clusters to explore the relationship between the three genotype groups graphically. The majority of the graphs showed a similar pattern of activation among L-allele carriers. While these results are unexpected, it is not clear whether many published studies of 5-HTTLPR genotype have explored multiple analytic models or extracted data to examine the fit of the model to the three genotype groups. Of the studies that have clearly considered the genotype groups individually, some found no differences between individuals with the LL and LS genotypes in hypothalamic-pituitary-adrenal reactivity in response to a laboratory stressor (
51) or in the likelihood of developing depression in response to moderately threatening life events (
52). As suggested by Gotlib et al. (
51), a single laboratory stressor may be too transient to elicit significantly different physiological reactivity between LL and LS carriers. Along with the present findings, this suggests that individuals with the SS genotype may have a lower threshold for stress sensitivity than their L-allele counterparts. However, the fact that these analyses were post hoc is a limitation, and further investigation is needed.
Recently, some controversy has arisen in the literature on the 5-HTTLPR-by-stress interaction because not all studies have replicated this gene-by-environment effect (
13,
53). Importantly, of those studies that did not replicate the effect, nearly all relied on retrospective questionnaire assessments of life stress exposure. Conversely, studies that utilized interview-based methods and rich multisource objective data in carefully followed epidemiological cohorts consistently found evidence of this gene-by-environment effect (
7,
8,
54,
55). This further highlights the utility of employing laboratory-based stressors, particularly when examining neural mechanisms mediating this effect. Moreover, in contrast to previous imaging genetics studies that relied on presentation of pictures acting as conditioned stimuli, the task we used provides an immediate and robust psychological and bodily threat, which is a visceral model of real-world stressors. As the field moves forward, it will be important to shift toward the type of paradigms that can powerfully unmask genetic sensitivity to threat and stress, such as the one used in this study.
Our results add to the mounting evidence, accumulated in studies of both animals and humans, suggesting that the S allele renders individuals stress-sensitive by biasing neurobiological systems underlying threat reactivity and arousal.
Despite its promise, this study has several limitations. First, while we prioritized studying a relatively homogeneous sample in order to decrease error variance, this sampling decision limits the generalizability of our findings. Future studies should examine neural mechanisms of genetic sensitivity to acute stress in men and within a wider range of genetic backgrounds. Second, while focusing on a healthy population allowed us to investigate the mechanism of risk without the confound of current or past psychopathology, it will be important for future studies to longitudinally follow participants from this type of imaging genetics experiment in order to determine whether these neural biomarkers result in the onset of psychopathology in the face of exposure to life stressors. Third, our small sample size limited power to detect all effects of genotype, particularly any effect on the amygdala (
15). Studies of larger samples are needed. Fourth, while research suggests that recent stressors are the most potent predictor of increased risk for psychopathology (
56) and hence this anxiogenic task provides a proxy for such acute real-life stressors, this design does not allow for investigation of the effects of chronic stressors or the full range of life stressors known to trigger psychopathology. Indeed, it may be the accumulation of chronic stress exposure over time that interacts with genotype to confer risk. Finally, our examination of brain-behavior relations is suggestive, but the directionality of these associations cannot be inferred; it could be that neural reactivity drives behavior or vice versa.