Suicide prevention strategies outlined by the Centers for Disease Control and Prevention (CDC), the U.S. Department of Health and Human Services, and the National Institutes of Health depend on establishing the frequency and severity of suicidal behavior and identifying risk and protective factors (
1,
2). Data collection to support these aims must employ valid and reliable assessment tools that allow comparison at local, national, and international levels.
A lack of uniformity in assessment stems in part from the variability of terms referring to similar or even identical behavior. Without a common language for reporting and communicating occurrences of suicidal behavior and ideation, prevention research is undermined in several key areas, including the establishment of reliable incidence and prevalence rates; comparison of data across studies, time periods, and locations; development of adequate tools for identifying and screening patients in primary and other care settings; measurement of risk and benefit in drug trials and postmarketing surveys; and training of health care workers, gatekeepers, and first responders in suicide risk detection (
3). Furthermore, the exclusion of individuals with suicidal ideation and behavior from clinical trials has resulted in a dearth of evidence for interventions for these populations, and the methods for determining which patients will be excluded have been variable, making it even more difficult to conduct analyses of these populations on suicide-related questions (
4,
5).
Studies of risk factors predicting suicide consistently suggest that suicidal ideation and a history of suicide attempts are among the most salient risk factors for suicide (
6–
9). Moreover, a structured assessment of suicidal ideation and behavior significantly improves identification of high-risk patients relative to a routine clinical interview (
10). However, to date, the field has lacked a single standard measure that assesses both suicidal ideation and behavior (
10).
To address inconsistencies in nomenclature, the impact of such inconsistencies on accurate identification, and the need for a single measure to assess the severity of and track changes in suicidal ideation and behavior, a team of investigators from Columbia University, the University of Pennsylvania, and the University of Pittsburgh developed the Columbia–Suicide Severity Rating Scale (C-SSRS).
Suicidal ideation and behavior have traditionally been conceived as a unidimensional construct, with passive ideation, active intent, and behavior existing along a continuum (
11,
12). The C-SSRS, however, was designed to distinguish the domains of suicidal ideation and suicidal behavior. Four constructs are measured. The first is the severity of ideation (hereafter referred to as the “severity subscale”), which is rated on a 5-point ordinal scale in which 1=wish to be dead, 2=nonspecific active suicidal thoughts, 3=suicidal thoughts with methods, 4=suicidal intent, and 5=suicidal intent with plan. The second is the intensity of ideation subscale (hereafter referred to as the “intensity subscale”), which comprises 5 items, each rated on a 5-point ordinal scale: frequency, duration, controllability, deterrents, and reason for ideation. The third is the behavior subscale, which is rated on a nominal scale that includes actual, aborted, and interrupted attempts; preparatory behavior; and nonsuicidal self-injurious behavior. And the fourth is the lethality subscale, which assesses actual attempts; actual lethality is rated on a 6-point ordinal scale, and if actual lethality is zero, potential lethality of attempts is rated on a 3-point ordinal scale.
The items for assessing severity of ideation (e.g., a specific plan or method) and intensity (e.g., frequency, duration) of ideation were based on factors predicting attempts and suicide identified in previous studies (
13–
20). The C-SSRS uses different assessment periods, depending on research or clinical need; the lifetime period assesses the worst-point ideation, which research has suggested may be a stronger predictor of subsequent suicide than current ideation (
7,
21).
Item selection for the scale was influenced by research on what aspects of past suicidal ideation and behavior predict the risk of future suicidal behavior, including severity of worst-point ideation and intent and medical damage or lethality of past suicide attempts (
7). Items assessing suicidal behavior were expanded to encompass not only actual attempts but also interrupted attempts, because these are predictive of suicide (
22), and aborted attempts, because they are associated with actual attempt behavior (
23). Preparatory activity was included in the assessments because analyses suggest that individuals who engage in preparatory behavior are more likely to commit suicide than those who do not report such behavior (
7,
23,
24). Neurobiological research suggests that the degree of suicide intent and the degree of medical lethality are related to serotonin indices in the brain (
25), which prompted the inclusion of items on the scale related to preparation for suicide attempt and to scoring the severity of medical damage. The more medically damaging or potentially lethal but nonfatal suicide attempts show serotonin abnormalities analogous to those found in postmortem examination of brain tissue from people who died by suicide (
26).
The C-SSRS was designed to 1) provide definitions of suicidal ideation and behavior and nonsuicidal self-injurious behavior and corresponding probes; 2) quantify the full spectrum of suicidal ideation and suicidal behavior and gauge their severity over specified periods; 3) distinguish suicidal behavior and nonsuicidal self-injurious behavior; and 4) employ a user-friendly format that allows integration of information from multiple sources (e.g., direct patient interview, family and other interviews, and medical records). As reviewed by Meyer et al. (
27), these criteria are considered essential for judging the utility of scales assessing suicide-related phenomena, and the scale is unique among rating instruments in meeting all of these criteria.
The C-SSRS (available at
www.cssrs.columbia.edu) includes definitions of suicidal behavior adapted from the Columbia Suicide History Form (
28). The definitions of ideation and behavior were also used in the Columbia Classification Algorithm for Suicide Assessment, commissioned by the U.S. Food and Drug Administration to classify retrospective reports of potentially suicidal adverse events and to provide interpretable data to inform pivotal drug safety questions (
29). These definitions were subsequently adopted by the CDC (
2).
In this study, we evaluated the C-SSRS's convergent, divergent, and predictive validity, its sensitivity and specificity, and its sensitivity to change, as well as the internal consistency of the intensity subscale, based on use of the scale in three multisite studies. Other indices of reliability could not be examined because of the study design, although interrater reliability has been demonstrated elsewhere (
30–
32).
Method
The studies used in the analyses are described below. More detailed descriptions of the studies and the relevant scales are included in a data supplement that accompanies the online edition of this article. Convergent validity, divergent validity, sensitivity, specificity, sensitivity to change, predictive and incremental validity, and internal consistency, as defined in
Table 1, were analyzed as shown in
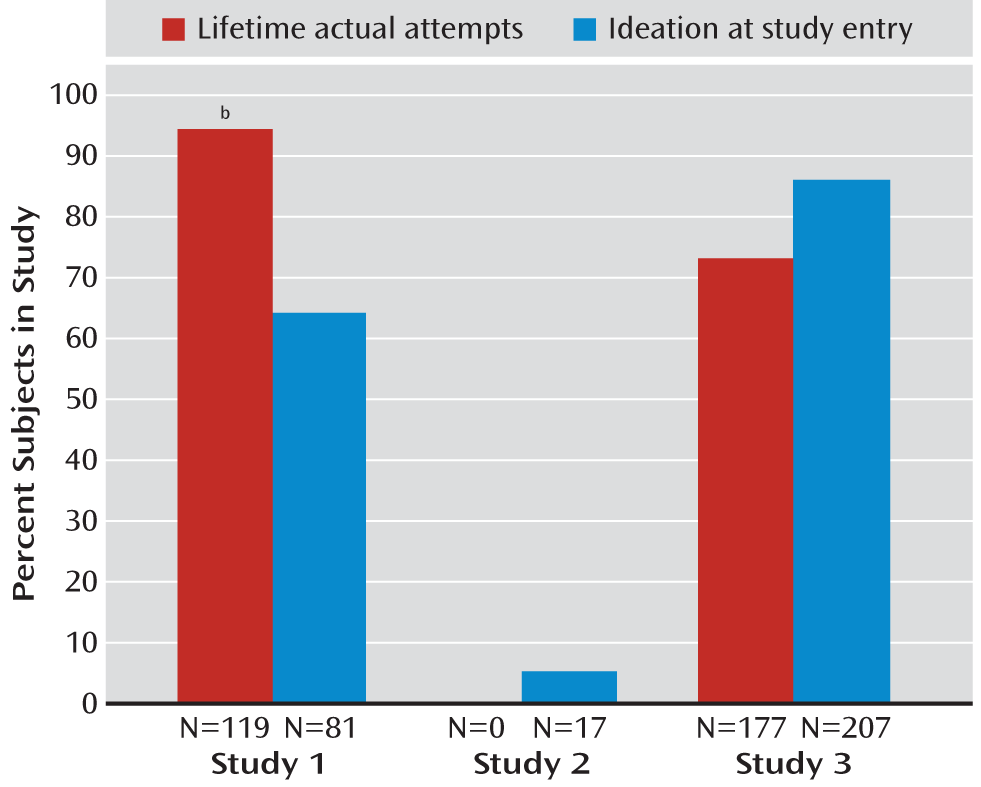
Table 2. Rates of suicidal ideation at study entry and lifetime actual suicide attempts for the three studies are presented in
Figure 1.
Studies Used in the Analyses
Study 1.
The Treatment of Adolescent Suicide Attempters study was a National Institute of Mental Health multisite feasibility study designed to develop and evaluate treatments to prevent suicide reattempts in adolescents. Participants were 124 male and female patients 12–18 years of age with a suicide attempt or interrupted attempt during the 90 days before enrollment (
34–
36). Participants were evaluated at baseline and at treatment weeks 6, 12, 18, and 24, as well as during intervening unscheduled visits. Evaluations included the C-SSRS, the Columbia Suicide History Form, the Scale for Suicide Ideation, and Beck's Lethality Scale. All instruments were administered by independent evaluators, who were Ph.D.-, R.N.-, or master's-level clinicians. Assessment of participants also included the self-report Beck Depression Inventory (BDI) at the same visits, as well as ratings by the treating psychopharmacologist (who was not the independent evaluator) on the Montgomery-Åsberg Depression Rating Scale (MADRS). Any potential suicidal events in the study were rated by the suicide evaluation board, which was an independent panel of suicidology experts uninvolved in the day-to-day management of the trial. The board, which was blind to original event classifications, treatment status, and other potentially biasing information, rated narratives according to predetermined criteria and definitions of potential suicidal events. Unanimous consensus was reached in cases where there was any initial disagreement.
Most participants (N=96, 77.4%) were assessed at week 12; 87 (70.2%) were evaluated at week 18, and 83 (66.9%) at week 24. Attrition between the study visits was due to participants refusing to continue study treatment or assessments. Participants who refused treatment but continued with assessments were included in the analyses. There was one death by suicide in the study during the follow-up period. As previously reported (
36), participants who remained in the study for longer than the median duration were similar to those who were followed for less than the median duration on all baseline predictors of suicidal events except income.
Study 2.
Study 2 was an industry-sponsored multisite, double-blind, placebo-controlled, parallel-group, fixed-dose clinical trial to evaluate the efficacy of the selective serotonin reuptake inhibitor escitalopram relative to placebo in the treatment of major depressive disorder (
37). Participants were 312 adolescents 11–17 years of age with a diagnosis of major depressive disorder. The study excluded patients who were considered a “suicide risk” by the investigators, including those who had suicidal ideation or had made a suicide attempt, although approximately 5.5% reported some level of ideation at study entry (
Figure 1).
The self-report Suicidal Ideation Questionnaire–Junior (the version for grades 7–9) (
38) and the C-SSRS were administered by the study clinicians at initial screening, at baseline, and at weeks 4, 8, 16, and 24 (or on early termination). Patients who completed at least one postbaseline assessment were included in the present analysis (N=259). The characteristics of the validation sample were similar to the baseline characteristics of the total sample (
37).
Study 3.
Study 3, funded by the American Foundation for Suicide Prevention, evaluated the identification and classification of recent suicide attempts and nonsuicidal self-injurious behavior by emergency department providers at three sites. Participants (N=237) were at least 18 years of age and presented to an emergency department for psychiatric reasons. They were categorized as having made a suicide attempt prior to the emergency evaluation, having engaged in nonsuicidal self-injurious behavior in the week prior to the evaluation, or reporting psychiatric symptoms without a suicide attempt or nonsuicidal self-injurious behavior prior to the evaluation. Study clinicians administered measures in the emergency department, including the C-SSRS, the Scale for Suicide Ideation, Beck's Lethality Scale, and the Columbia Suicide History Form.
Data Analyses
Analyses of data from studies 1 and 2 were conducted with SAS, version 9.1 (SAS Institute, Cary, N.C.). Analyses of data from study 3 were conducted with SPSS, version 19 (SPSS, Chicago).
Convergent and divergent validity.
In study 1, to examine the convergent and divergent validity of the ideation and behavior components of the C-SSRS, associations between the scale and other instruments measuring corresponding constructs were initially tested using Pearson's r. Effect sizes were computed (Cohen's d=2r/sqrt[1–r2]) in order to compare the magnitude of the correlations. A mixed-effects regression model was used to adjust for the effect of repeated assessments over the course of the study. The C-SSRS ideation scores were entered as dependent variables and the scores from corresponding scales as independent variables. Unstructured covariance structure was specified.
The choice of random effects was based on the following assumption: each participant has his or her own systematic baseline on the measures, and each starting point is treated as the result of a random deviation from some mean intercept. This model captures both the repeated-measures effect and random-intercept effect in individuals when the correlation between measures is assessed. Intercept and time (days in treatment) were included as random-effect variables. A Steiger's z test for comparison of correlated correlations evaluated whether the severity and intensity subscales were more related to the suicidal ideation item than items measuring somatic features of depression on the MADRS and the BDI.
In study 2, the analysis was parallel to that in study 1 except that the self-report Suicidal Ideation Questionnaire–Junior total score was used as the criterion measure for the convergent validity of the intensity subscale. The convergent and divergent validity of the C-SSRS behavior subscale and the lethality subscales were not examined in study 2 because no other behavior measure was available for comparison.
In study 3, the convergent validity of the severity subscale, intensity subscale, and behavior subscale was evaluated with the phi coefficient.
Sensitivity/specificity and sensitivity to change.
Analysis of specificity in studies 1 and 3 examined the rate of true negatives on the C-SSRS behavior subscale relative to behavior ratings on the Columbia Suicide History Form and, in study 1, also to the suicide evaluation board ratings. The rate of true positives relative to the ratings on the Columbia Suicide History Form and the suicide evaluation board ratings was used to show the sensitivity of the behavior subscale.
In studies 1 and 2, mixed-effects linear regression was used to test sensitivity to change of the severity and behavior subscales over the study period as measured by criterion scales. The C-SSRS scores were entered as dependent variables, and the scores from the Scale for Suicide Ideation (study 1) or the Suicidal Ideation Questionnaire–Junior (study 2) were entered as independent variables. Unstructured covariance structure was specified, and intercept and time (days in treatment) were included as random-effect variables.
Predictive and incremental validity.
In study 1, the predictive validity of the worst-point lifetime ideation on the severity subscale for 1) actual attempts and 2) actual, interrupted, and aborted attempts combined as reported on the Columbia Suicide History Form between weeks 1 and 24 was examined using logistic regression. Similarly, the predictive validity of the severity subscale was tested for the actual attempts classified by the evaluation board. The C-SSRS severity scores were entered as a continuous variable, and suicidal behavior, including multiple attempts, as a dichotomous variable. The average length of time between the baseline administration of the C-SSRS and the week 24 Columbia Suicide History Form ratings was 134.63 days (SD=65.48), as some subjects had their week 24 visit beyond 168 days. This variability was addressed in the model by including time as a covariate.
Incremental validity was evaluated by comparing the predictive validity of the lifetime worst-point ideation (including past week) on the C-SSRS severity subscale to the predictive validity of 1) the total score and 2) the suicidal intent items on the Scale for Suicide Ideation for the same time period. Because intent to die appears to confer heightened risk for suicide (
14,
15,
39), it was hypothesized a priori that presence of ideation with at least some intent (the two most severe levels of ideation on the C-SSRS) would confer greater risk than presence of ideation without intent. Logistic regression was used to test whether a history of ideation with at least some intent in contrast to levels of ideation on the C-SSRS without intent resulted in a greater risk of suicide attempts or combined ratings of aborted, interrupted, and actual attempts classified on the Columbia Suicide History Form and actual attempts classified by the evaluation board.
Internal consistency.
The internal consistency of the C-SSRS ideation subscale in all three studies was tested with Cronbach's alpha. The severity and behavior subscales use an ordinal scale and are therefore not subject to internal consistency analysis. Other types of reliability (e.g., interrater) could not be examined because of the design of the studies.
Results
Convergent Validity
At baseline, the C-SSRS severity subscale and the Scale for Suicide Ideation assessment of worst-point ideation both capture the most suicidal period during the patient's lifetime; at all subsequent assessments, the since-last-visit and worst-point assessments both measure the most severe suicidal ideation since the last assessment.
The C-SSRS severity subscale was moderately correlated with the worst-point score on the Scale for Suicide Ideation (r=0.52, p<0.001; effect size=1.22, N=472). The C-SSRS intensity subscale containing the frequency, duration, controllability, certainty, and deterrents items for the most severe ideation was moderately correlated with the worst-point ideation total score on the Scale for Suicide Ideation (r=0.56, p<0.001; effect size=1.36, N=487). For the since-last-visit assessments, there was a strong relationship between the C-SSRS severity subscale and the MADRS suicidal ideation item (r=0.63, p<0.001; effect size=1.61) as well as the BDI suicide item, which asks subjects to rate on a 4-point scale their thoughts of killing themselves (r=0.80, p<0.001; effect size=2.66) (
Table 3). For the since-last-visit assessments, there was also a strong relationship between the C-SSRS intensity subscale and the MADRS suicidal ideation item (r=0.69, p<0.001; effect size=1.93) as well as the BDI suicide item (r=0.51, p<0.001; effect size=1.19).
In study 2, the convergent validity of the C-SSRS with the self-report Suicidal Ideation Questionnaire–Junior total score over the course of the study was moderate for the C-SSRS severity subscale (r=0.36, p<0.01; effect size=0.77) and low for the C-SSRS intensity subscale (r=0.23, p<0.001, effect size=0.47) based on pair correlations at multiple time points across the study (total number of pairs=234).
In study 3, the correlation between the C-SSRS severity subscale and the Scale for Suicide Ideation total score was moderate (r=0.69, p<0.001, N=211). The C-SSRS intensity subscale total score correlated modestly with the Scale for Suicide Ideation total score (r=0.34, p<0.001; N=193).
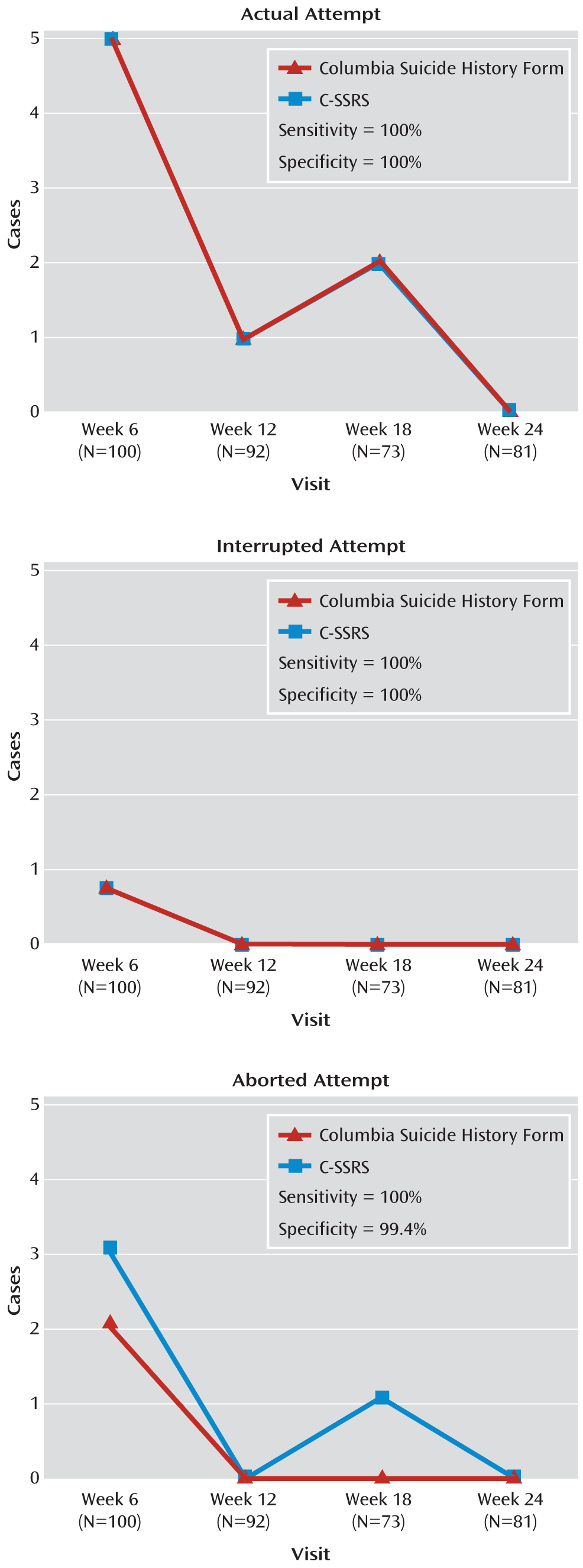
In study 1, the C-SSRS's sensitivity and specificity of behavior classifications relative to the behavior classifications on the Columbia Suicide History Form and those by the evaluation board were examined. A total of 15 study subjects had at least one actual, interrupted, or aborted attempt. The total number of these three behaviors in the study based on all visits was 24, including unscheduled (emergency) visits. Relative to the Columbia Suicide History Form, the C-SSRS had 99.4% specificity and 100% sensitivity in correctly identifying aborted attempts and 100% sensitivity and specificity for both interrupted and actual attempts (
Figure 2). Two aborted attempts were identified by the behavior subscale that were not identified using the Columbia Suicide History Form. The C-SSRS demonstrated 100% sensitivity and 96% specificity relative to the evaluation board ratings using the combined classifications of actual and interrupted attempts. Five cases of behavior were identified on the behavior subscale but not by the evaluation board. Because the board did not classify aborted attempts (of which two were identified by the C-SSRS), the total number of attempts analyzed here is 22, not 24.
In study 2, the convergent validity of behavior classifications was not examined because no parallel measure of suicidal behavior was available.
In study 3, the degree of association regarding the identification of lifetime (including past week) actual, interrupted, and aborted attempts using the C-SSRS and the Columbia Suicide History Form was high (phi values, 0.99, 0.92, and 0.94, respectively; all p values <0.001; N=237). The C-SSRS had 100% specificity and 100% sensitivity in correctly identifying lifetime actual attempts and 99% specificity and 94% sensitivity in correctly identifying lifetime interrupted attempts that were recorded on the Columbia Suicide History Form. Two lifetime interrupted attempts were identified on the Columbia Suicide History Form that were not identified using the C-SSRS. The scale had 99% specificity and 93% sensitivity in correctly identifying lifetime aborted attempts that were recorded on the Columbia Suicide History Form. Four lifetime aborted attempts were identified on the Columbia Suicide History Form that were not identified using the C-SSRS.
The actual lethality subscale was robustly correlated with the Beck's Lethality Scale score from the Columbia Suicide History Form (r=0.79, p<0.001; N=237). The lethality subscale is rated on a 6-point scale (running from 0 to 5) using general anchors for lethality, whereas the Columbia Suicide History Form lethality subscale is rated on a 10-point scale (running from 0 to 9) using anchors that correspond to the method of injury.
Divergent Validity
In study 1, the divergent validity of the C-SSRS severity subscale was examined by correlating raw scores on the fatigue, sleep, appetite, and loss of energy items on the self-report BDI and similar items on the MADRS, administered by the psychopharmacologist, with the C-SSRS's most severe ideation since last visit, administered by a different clinician. Effect sizes using Cohen's d were computed to evaluate the strength of the significant correlations. As in the analyses of convergent validity, mixed-effects regression was used to adjust for the effect of multiple assessments over time (
Table 3).
For the since-last-visit assessments, weak or moderate correlations were observed between the C-SSRS severity and intensity subscales and the BDI and MADRS somatic depression items. A much stronger association with the BDI and MADRS suicidal ideation items, in contrast to the weak relationship with nonsuicidal items demonstrated by the Steiger's z test for comparison of correlated correlations, further supports the divergent validity of the C-SSRS (
Table 3). When adjusted for the effect of multiple assessments, the findings were similar—for example, a one-unit increase in the severity subscale score for the since-last-visit assessments correlated (on average) with 0.278 units on the BDI sleep disturbance item (
Table 3).
The relationship of the C-SSRS intensity subscale total score to somatic symptoms of depression on the BDI and MADRS was also examined. As seen in
Table 3, correlations between the intensity subscale and the BDI and MADRS items were generally small to moderate, in contrast to larger correlations with the C-SSRS severity subscale. Effect sizes for these associations varied from small to large. When adjusted for the effect of repeated assessments, a one-unit change on the BDI and MADRS scores expressed in standard deviation units corresponded to measurable changes in the intensity subscale, indicated by the beta coefficients.
The divergent validity of the C-SSRS severity and intensity subscales in studies 2 and 3 was not examined.
Sensitivity to Change
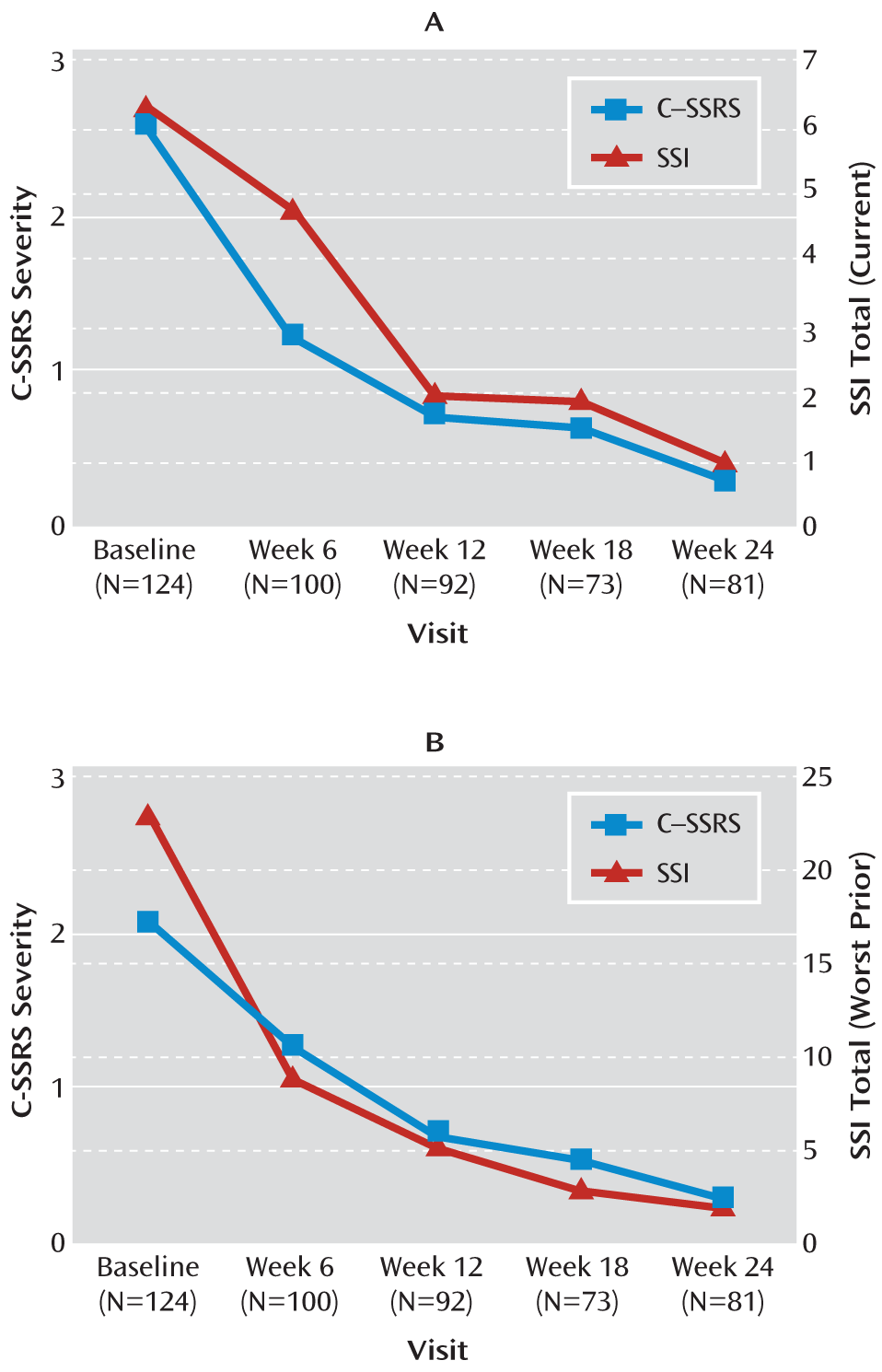
In study 1, results from the random-effects mixed linear regression that tested sensitivity to change of the C-SSRS on the criterion scales over the study period showed that the scale's ideation and behavior ratings were related to other measures over time. In the linear regression, a one-unit decrease in the “total current” ideation score on the Scale for Suicide Ideation corresponded to a decrease of 0.106 units in the C-SSRS severity subscale score (p<0.001). Similarly, change in the Scale for Suicide Ideation worst-point ideation corresponded to a significant change on the C-SSRS intensity subscale for since-last-visit assessments (beta=0.071, p<0.001).
Figure 3 demonstrates that the mean severity subscale scores and the mean intensity subscale scores responded similarly to the change on the Scale for Suicide Ideation. All unscheduled/emergency visits (N=39) occurring between baseline and week 24 were excluded from these analyses.
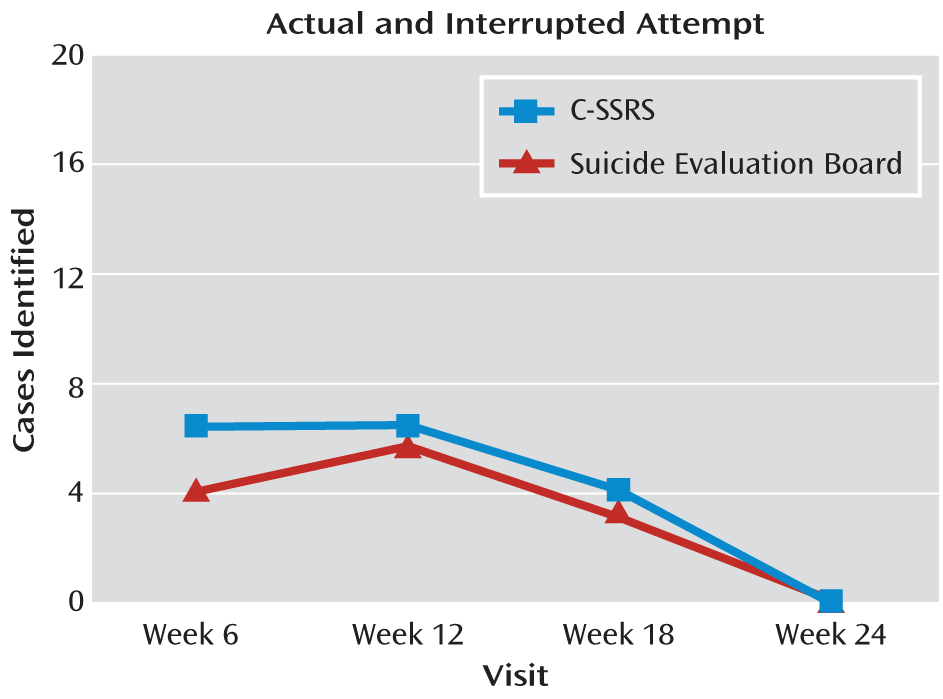
To assess the behavior subscale's sensitivity to change, three types of suicidal behaviors (aborted, interrupted, and actual attempts) identified by the independent evaluators were plotted against those identified by the Columbia Suicide History Form at weeks 6, 12, 18, and 24 (
Figure 2) and against interrupted, actual, and one completed attempt combined, as classified by the evaluation board. There was full agreement for interrupted and actual attempts and moderate agreement for aborted attempts relative to the Columbia Suicide History Form classifications (kappa=0.66, 95% confidence interval [CI]=0.23–1.00) and high agreement (kappa=0.88, 95% CI=0.77–0.98) with the attempt and interrupted attempt classifications made by the evaluation board (
Figure 4). Only cases in which both the C-SSRS and Columbia Suicide History Form ratings (N=364) from the same assessments were available were included in the analysis. All unscheduled/emergency visits occurring between baseline and week 24 were excluded from the analysis. All potential events on the C-SSRS were later classified by the evaluation board and included in the analyses. One suicide rated by the board after the study was completed was not rated on the scale.
In study 2, linear regression showed that a one-unit decrease in total score on the Suicidal Ideation Questionnaire–Junior corresponded to an average decrease of 0.31 units in the C-SSRS severity subscale score (p=0.01) and to a decrease of 0.17 units in the intensity of ideation scores (p<0.001).
Predictive and Incremental Validity
The predictive validity of the C-SSRS was evaluated using the Columbia Suicide History Form and the suicide evaluation board classifications; the severity subscale was examined continuously and categorically (with or without intent). The results were comparable with either approach.
In study 1, baseline C-SSRS ratings (based on worst-point lifetime suicidal ideation and conducted before patients received any treatment) significantly predicted suicide attempts during treatment (odds ratio=1.45, 95% CI=1.07–1.98, p=0.02) as well as actual, interrupted, and aborted attempts combined on the Columbia Suicide History Form between week 1 and week 24 (odds ratio=1.34, 95% CI=1.05–1.70, p=0.02) and classifications made by the suicide evaluation board. In the parallel analyses predicting the behavior classifications made by the suicide evaluation board from the baseline severity of ideation ratings, for a one-standard-deviation increase in the lifetime (including past week) severity of ideation on the C-SSRS, the odds of actual attempts increased by 43% (odds ratio=1.43, 95% CI=0.99–2.05, p=0.05). Analyses included all 124 participants who were assessed at baseline, using intent-to-treat model.
For every one-standard-deviation increase in the level of lifetime suicidal ideation reported at study entry, the odds of attempting suicide during the study increased by 45%. The odds of actual, interrupted, or aborted suicide attempts combined as assessed by the Columbia Suicide History Form increased by 34% for every one-standard-deviation increase in lifetime severity of ideation score. Predictive validity was also evaluated using a lifetime history of the most severe ideation with and without intent on the C-SSRS severity subscale. The odds of suicide attempts scored on the Columbia Suicide History Form during follow-up were compared in participants who endorsed a lifetime history of the two most severe levels of ideation (where at least some intent to die was present) reported at baseline and those who endorsed a history of less severe ideation (types 0–3) (odds ratio=3.26, 95% CI=1.02–10.45, p=0.047). The lifetime history of the two highest severity levels of ideation reported on the C-SSRS at baseline were also associated with higher odds of actual, interrupted, and aborted attempts combined on the Columbia Suicide History Form (odds ratio=2.76, 95% CI=1.07–7.12, p=0.036). In the parallel analyses, predicting from the dichotomously scored lifetime severity of ideation to the behavior classifications by the evaluation board, the odds of actual attempts were almost four times higher for those with a lifetime history (including past week) of the two most severe levels of ideation at baseline (odds ratio=3.85, 95% CI=1.07–13.86, p=0.039).
We conducted similar analyses of predictive validity with the total score (lifetime) and with the intent items of the Scale for Suicide Ideation. No significant results were observed for any of the outcomes. A one-standard-deviation increase in the total score (lifetime) was not associated with an increase in odds of suicide attempts on the Columbia Suicide History Form (odds ratio=1.02, 95% CI=0.95–1.11, p=0.57) or in odds of actual, interrupted, and aborted attempts combined on the Columbia Suicide History Form at follow-up (odds ratio=1.01, 95% CI=0.95–1.07, p=0.79).
In the categorical analysis based on the intent items (a score ≥1 on item 4 or 5) from the Scale for Suicide Ideation, the odds of attempts during the study were not higher for actual attempts (odds ratio=1.44, p=0.76; all of the attempters endorsed intent) or for the combined ratings of actual, interrupted, and aborted attempts (odds ratio=2.19, p=0.49; all of the attempters endorsed intent).
Because baseline Scale for Suicide Ideation scores (either total score or score on the intent items) were not significantly associated with attempts during the study, the incremental predictive validity of the C-SSRS severity subscale relative to the Scale for Suicide Ideation could not be estimated.
Internal Consistency
In study 1, the C-SSRS intensity subscale was examined at two assessment intervals: since last visit and past week. The internal consistency of the intensity subscale was high, with a Cronbach's alpha of 0.937 for since last visit and 0.946 for the past week. In studies 2 and 3, the internal consistency of the intensity subscale across all visits was moderate, with a Cronbach's alpha of 0.73.
Discussion
We examined the psychometric properties of the C-SSRS and demonstrated convergent, divergent, and predictive validity; sensitivity to change; sensitivity and specificity of the instrument; and internal consistency of the intensity subscale. The study design precluded examination of other indices of reliability, although interrater reliability has already been demonstrated (
31,
32,
36). In all three studies analyzed, the C-SSRS ideation and behavior subscales showed strong convergent validity with established ideation and behavior scales. The finding of only a moderate convergent relationship between the C-SSRS severity and intensity of ideation scores and the Scale for Suicide Ideation total score, as well as the Suicidal Ideation Questionnaire–Junior score, was expected and was likely due to nonoverlap in items and different construct operationalization. The C-SSRS demonstrated strong divergent validity with items on the BDI and the MADRS that were not expected to overlap with suicidal ideation and behavior (e.g., somatic depression symptoms such as fatigue). Because suicide risk and protective factors are not orthogonal to psychiatric diagnosis (
40), establishing divergent validity with measures of other constructs is essential. In suicidal adolescents, ideation was most closely related to suicide-related items in other measures, in contrast to other types of symptoms of psychiatric disorders or physical illnesses.
Several other findings have important implications. In study 1, a decrease in the severity of ideation from “active suicidal thoughts” to “wish to die” or “no ideation” was accompanied by corresponding decreases in Scale for Suicide Ideation score, suggesting that the C-SSRS severity subscale is sensitive to clinical change. Similarly, the C-SSRS identified almost an identical number of cases with specific types of suicidal behavior compared to the Columbia Suicide History Form and the suicide evaluation board ratings, and this agreement held over the course of the study. There was a high degree of agreement in the classification of suicidal behavior between the Columbia Suicide History Form, the evaluation board ratings, and the C-SSRS. The behavior subscale demonstrated high sensitivity and specificity relative to behavior classifications on the Columbia Suicide History Form and by the evaluation board.
A striking finding in this study was that the C-SSRS demonstrated predictive validity but the Scale for Suicide Ideation did not. The Scale for Suicide Ideation has shown predictive validity for death by suicide with adults in other long-term follow-up studies (
41,
42), but it did not predict near-term nonfatal suicidal behavior in this study. As with any cross-study comparison, differences in predictive validity may result from differences in clinical populations, assessment context, and time. Another plausible explanation for this divergent finding is scoring differences between the two scales. The Scale for Suicide Ideation total score aggregates many characteristics of suicidal ideation along a continuum, whereas the C-SSRS identifies types of ideation and classifies individuals as having intent, as well as having intent and a plan. Factor structure analyses of the Scale for Suicide Ideation have shown that certain clusters of items assessing “plans” and “desire” show differential prediction of past attempts and eventual suicide (
21,
43). The present study raises the question of whether identifying specific types of suicidal ideation may be more useful for prospective research and for risk stratification. Further prospective studies of suicide risk using standardized measures are warranted.
Of particular interest are the results from study 1 suggesting that a history of severe ideation with at least some intent to die may confer a greater risk for suicidal behavior than a history of ideation with no intent to die. Establishing clinically meaningful thresholds that indicate heightened suicide risk and predict which individuals will go on to engage in suicidal behavior have been elusive in efforts at suicide prevention (
3). Because ideation severity can be used to set criteria for clinical referral and inclusion or exclusion criteria in research, operationalization of clinical thresholds could facilitate enrollment of patients with suicidal behavior in research studies, as well as clinical management more broadly.
As with any instrument, one must be mindful of the population one is working with. Depending on the population, 25% to 60% of attempts are considered “impulsive” (
44–
46), but the role of impulsivity in suicide is far from clear (
41,
42), and “impulsive” attempts may include prior ideation and planning (
41). The results should be replicated in studies designed to test psychometric properties of the scale, and the application of the scale in different populations deserves further study, particularly in light of the different potential routes to suicidal behavior (e.g., which severity items predict which behaviors, and what other factors, such as substance abuse or family history, modify the risk associated with the severity or intensity of ideation).
Limitations
There are several significant limitations to this analysis. The studies on which our analyses are based were not prospectively designed to examine psychometric properties of the instruments, although the replication of findings across three different studies is reassuring. The significant associations between the C-SSRS and the Scale for Suicide Ideation and the Columbia Suicide History Form may be attributed in part to the fact that the same interviewers contemporaneously administered the scales; while this may be an advantage in demonstrating convergent validity, it also increases the risk of interviewer or rater bias. To address this issue, self-report measures (the BDI and the Suicidal Ideation Questionnaire–Junior), ratings (on the MADRS) by a study psychopharmacologist who was not administering the C-SSRS, and ratings by the independent suicide evaluation board were analyzed, and the results were similar. Nevertheless, further examination of concurrent and incremental validity and interrater reliability of this scale with other measures of suicidal ideation and behavior is warranted.
The incidence of aborted, interrupted, and actual attempts was very low in the two prospective studies (studies 1 and 2), which limits the precision of the sensitivity and specificity estimates. The generalizability of the predictive validity findings is limited in study 1 because only adolescent attempters were included in the sample (thus, predictive validity for that study refers to prediction of reattempt). Although there are advantages to using diverse populations in these analyses, there may be limitations to the generalizability of results. While study 1 included only adolescent attempters, study 2 excluded individuals with current ideation or a history of suicide attempt, and study 3 was a clinical sample of adults presenting to an emergency department for psychiatric treatment. These findings must be replicated with community samples.
Conclusions
These analyses of the C-SSRS using data from three studies provide initial promising data on the convergent and divergent validity, predictive validity, sensitivity, specificity, sensitivity to change, and internal consistency of the C-SSRS. Greater precision in the assessment of suicidal behavior and ideation is necessary for improving identification and clinical management, as well as for research-derived risk-benefit analyses. The use of a standardized measure such as the C-SSRS that comprehensively assesses suicidal behavior and ideation permits comparison of findings across research and clinical populations, as well as trends over time, providing data to guide treatment recommendations for suicidal patients and suicide prevention efforts.