Attention-deficit hyperactivity disorder (ADHD), one of the most common and debilitating childhood disorders, is defined in DSM-IV-TR as a disorder of age-inappropriate impulsiveness, inattention, and hyperactivity. ADHD persists into adulthood in 65% of cases (
1). Cognitively, ADHD patients have deficits in higher-level executive, attention, and timing functions that are known to be mediated by late-developing fronto-striatal and fronto-parietal/temporal networks (
2).
Neuroimaging studies over the past decade have provided consistent evidence that the behavioral and cognitive deficits of ADHD are associated with underlying structural and functional brain abnormalities. For example, structural MRI studies using region-of-interest analyses have reported abnormal volume and cortical thickness in several brain regions (
3), including total brain volume (
4), the corpus callosum (
5), prefrontal regions (
4,
6), the basal ganglia (
4,
7), and the temporal and parietal cortices (
8,
9). Longitudinal studies have shown that children with ADHD show a delay in the peak of cortical thickness maturation by 3–5 years, with the greatest delays in frontal and temporal brain regions (
8). Diffusion tensor imaging studies have shown abnormalities in a range of white matter tracts, which is thought to reflect a widespread dysmaturation of myelination and thus structural brain connectivity (
10). The findings of white matter tract deficits suggest that structural deficits in ADHD are not just confined to specific regions but affect the structural interconnectivity between regions and hence entire neural networks (
10).
Although there is evidence for structural brain abnormalities in pediatric ADHD, the findings of these studies have been relatively inconsistent, with several pediatric imaging studies reporting no group differences in total brain volume (
11,
12) or basal ganglia volume (
6,
7,
12). Furthermore, most studies have been conducted in pediatric populations. Structural imaging studies in adults with ADHD generally report gray matter and cortical thickness deficits in the same brain regions as those previously reported in childhood ADHD, such as the superior, inferior, and orbitofrontal brain regions, the anterior cingulate (
13,
14), the basal ganglia (
14,
15), the temporo-parietal regions (
13), and the cerebellum (
14). However, there have also been negative findings of no replication of abnormal gray matter volumes in frontal and striatal brain regions in adults with ADHD, raising the question of whether ADHD patients partially outgrow their structural brain abnormalities (
16–
18).
The recent development of fully automated whole-brain voxel-based morphometry methods (
19), which overcome the difficulties in the manual delimitation of discrete brain regions, has provided powerful tools for studying the potential changes in gray matter volume in ADHD. Unfortunately, recent applications of these novel methods to the study of gray matter volumetric changes in ADHD have been limited by relatively small sample sizes and thus insufficient statistical power. Ellison-Wright et al. (
20) conducted a meta-analysis of seven voxel-based morphometry studies of children with ADHD and found a significant gray matter deficit in the right putamen and globus pallidus. However, the analysis was limited by its use of only a small number of studies and of two nonclinical samples from a twin study (
21). Therefore, that first meta-analysis of voxel-based morphometry studies, although useful, may have been severely underpowered for examining structural changes in ADHD. Furthermore, the analysis did not include adult ADHD studies and hence could not elucidate consistent brain deficits across the lifespan or age-associated effects.
Another issue that remains unclear is the role of stimulant medication in structural brain changes in ADHD. While there are no prospective longitudinal randomized controlled studies investigating the long-term effects of stimulants on brain structure, several structural MRI studies have included retrospective comparisons between relatively small subgroups of patients with and without a history of stimulant medication matched on age, IQ, and symptom severity. The comparisons showed that medicated relative to medication-naive children with ADHD have more normal white matter overall (
22) and more normal gray matter volumes, cortical thickness, or morphometry in various brain regions typically associated with ADHD, including the inferior frontal, premotor, and parietal cortical areas (
23), the cerebellar vermis (
24), the anterior cingulate gyrus (
25), the caudate (
26,
27), and the thalamus (
28). Normalization findings in the basal ganglia are particularly relevant as they may reflect architectural modifications of this area by chronic methylphenidate use, which is known to block dopamine transporter levels and enhance dopamine availability, most prominently in the basal ganglia (
29). Although findings were cross-sectional and confounded by selection bias, the suggestion that stimulant medication may have a normalizing effect on brain structure in ADHD deserves further research. It is also possible that the inclusion of medicated patients may have affected previous MRI results and that heterogeneity in medication status between studies could potentially explain inconsistencies in the literature.
The goals of this study were therefore threefold: to establish the most prominent and replicable structural abnormalities in ADHD, to examine age-related effects on gray matter volume differences, and to explore whether stimulant medication is associated with more normal gray matter volumes in ADHD. We performed a voxel-based meta-analysis of published and unpublished gray matter voxel-based morphometry studies in pediatric and adult ADHD, as sufficient numbers of suitable studies are now available for analysis. We also applied novel multiple metaregression methods to explore potential age and medication effects. Finally, to facilitate replication and further analyses by other colleagues, we have also developed a readily accessible online database (
http:/www.sdmproject.com/database), which contains all the data and methodological details from every study included in this meta-analysis.
Method
Study Selection
A comprehensive literature search of studies conducting voxel-based morphometry comparisons between patients with ADHD and healthy comparison subjects published between 2001—the date of the first voxel-based morphometry study in ADHD—and May 2011 was conducted using the PubMed, ScienceDirect, Web of Knowledge, and Scopus databases. The search keywords were “attention-deficit hyperactivity disorder,” “ADHD,” or “hyperkinetic,” plus “morphometry,” “voxel-based,” or “voxel-wise.” In addition, we conducted manual searches in several review articles and the reference sections of the identified articles. We excluded studies that used duplicated data sets (i.e., studies that analyzed the same data in different articles), studies that included nonclinical samples, studies that did not compare patients and healthy comparison subjects, studies that examined the neural correlates of ADHD in individuals with chromosomal abnormalities, and studies that used fewer than 10 patients. The corresponding authors were invited by e-mail to provide additional details not included in the original manuscripts. Guidelines from the Meta-Analysis of Observational Studies in Epidemiology group (
30) were followed in the study.
Comparison of Global and Regional Gray Matter Volumes
Meta-analytical differences in global gray matter volumes were calculated using standard random-effects models with the Globals procedure in the Signed Differential Mapping (SDM) software package (
www.sdmproject.com), which uses restricted maximum-likelihood estimation of the variance, a fitting method that has been recommended over previous ones for its good balance between unbiasedness and efficiency (
31).
Regional differences in gray matter volume between patients and comparison subjects were also analyzed using SDM, which employs a voxel-based meta-analytic approach that is based on, and improves on, other existing methods (
32,
33). The main advantage of SDM is that it uses the reported peak coordinates to recreate maps of the signed (i.e., positive and negative) volume differences between patients and comparison subjects, rather than just assessing the probability or likelihood of a peak. This unique feature makes SDM an optimal method for comparing patients and comparison subjects without biasing the results toward those brain regions that show more interstudy heterogeneity. The SDM methods have been described in detail elsewhere (
32) and are briefly summarized here. First, peak coordinates of gray matter differences between patients and comparison subjects are extracted from each data set. Notably, those peaks that do not appear statistically significant at the whole brain level are excluded; that is, while different studies may employ different thresholds, we ensure that the same statistical threshold throughout the brain is used in each study. This is intended to avoid biases toward liberally thresholded brain regions, as it is not uncommon in neuroimaging studies for the statistical threshold for some regions of interest to be rather more liberal than for the rest of the brain. Second, a standard Talairach map of the differences in gray matter is separately recreated for each study by means of a Gaussian kernel, which assigns higher values to the voxels closer to peaks. This procedure includes limiting voxel values to a maximum to avoid biases toward studies reporting various coordinates in close proximity and reconstructing both increases and decreases of gray matter volume in the same map. Full width at half maximum of the kernel is 25 mm, to deal with variations in the size and shape of the original clusters, the position of the reported peak within the original clusters, or the spatial mismatch between the cortical structures of different subjects even after spatial normalization. Third, the mean map is obtained by voxel-wise calculation of the mean of the study maps, weighted by the squared root of the sample size of each study, so that studies with large sample sizes contribute more.
We also conducted additional reliability analyses to assess the robustness of the findings (
32): a jackknife sensitivity analysis to assess the reproducibility of the results and descriptive analyses of quartiles. The jackknife sensitivity analysis consists in iteratively repeating the same analysis, excluding one data set at a time to establish whether the results remain significant. The analyses of quartiles describe the proportion of studies reporting changes in a particular region (i.e., 25%, 50%, 75%), regardless of p values (
32,
33).
Statistical significance was determined using standard randomization tests, thus creating null distributions from which p values can be directly obtained (
32). Finally, we conducted a subgroup analysis of pediatric/adolescent and adult samples, a subgroup analysis of studies conducting the additional voxel-based morphometry modulation step, and a multiple metaregression analysis with age and the percentage of patients receiving stimulant medication in each study as regressors (
33).
Results
Included Studies and Sample Characteristics
The search yielded 17 studies. Three studies were discarded because they did not meet study criteria: one used a nonclinical sample (
21), one examined the volumetric correlates of ADHD symptoms in individuals with chromosome 22q11.2 deletion syndrome (
34), and one did not conduct comparative analyses between ADHD participants and healthy comparison subjects (
35). After we requested and received information from the authors of the remaining 14 studies, no methodological ambiguities remained regarding the design or analysis of any of the comparisons. Therefore, 14 high-quality data sets could be included in this meta-analysis, of which five (
14–
18) consisted of adult ADHD samples and the remaining nine (
36–
43; K. Rubia et al., unpublished 2010 data) of pediatric/adolescent samples.
Combined, the studies included 378 patients with ADHD and 344 healthy comparison subjects. Patients were 202 children/adolescents (12 hyperactive; 29 inattentive; 74 combined; 87 unknown) and 176 adults (82 with combined type ADHD; 94 with unknown type). As shown in
Table 1, no relevant differences between patients and comparison subjects were found in age, gender, or IQ, as the original studies were already well matched in this respect. Further details of each of the included studies, such as comorbid conditions, medication status, or diagnostic criteria, can be found at
www.sdmproject.com/database.
Global Differences in Gray Matter Volume
Global gray matter volumes were available from seven (six pediatric; one adult) independent data sets (
18,
36–
38,
40–
42). Patients with ADHD (N=191) had significantly smaller global gray matter volumes compared with healthy comparison subjects (N=179) (unbiased d=–0.28, z=–2.65, p=0.008). This difference was more pronounced when the adult study was excluded (unbiased d=–0.33, z=–2.93, p=0.003). No significant heterogeneity was found in either analysis (all data sets: Q=6.08, df=6, p=0.414; only pediatric data sets: Q=4.60, df=5, p=0.466).
Regional Differences in Gray Matter Volume
Data for this analysis were obtained from all the studies included in the meta-analysis. As shown in
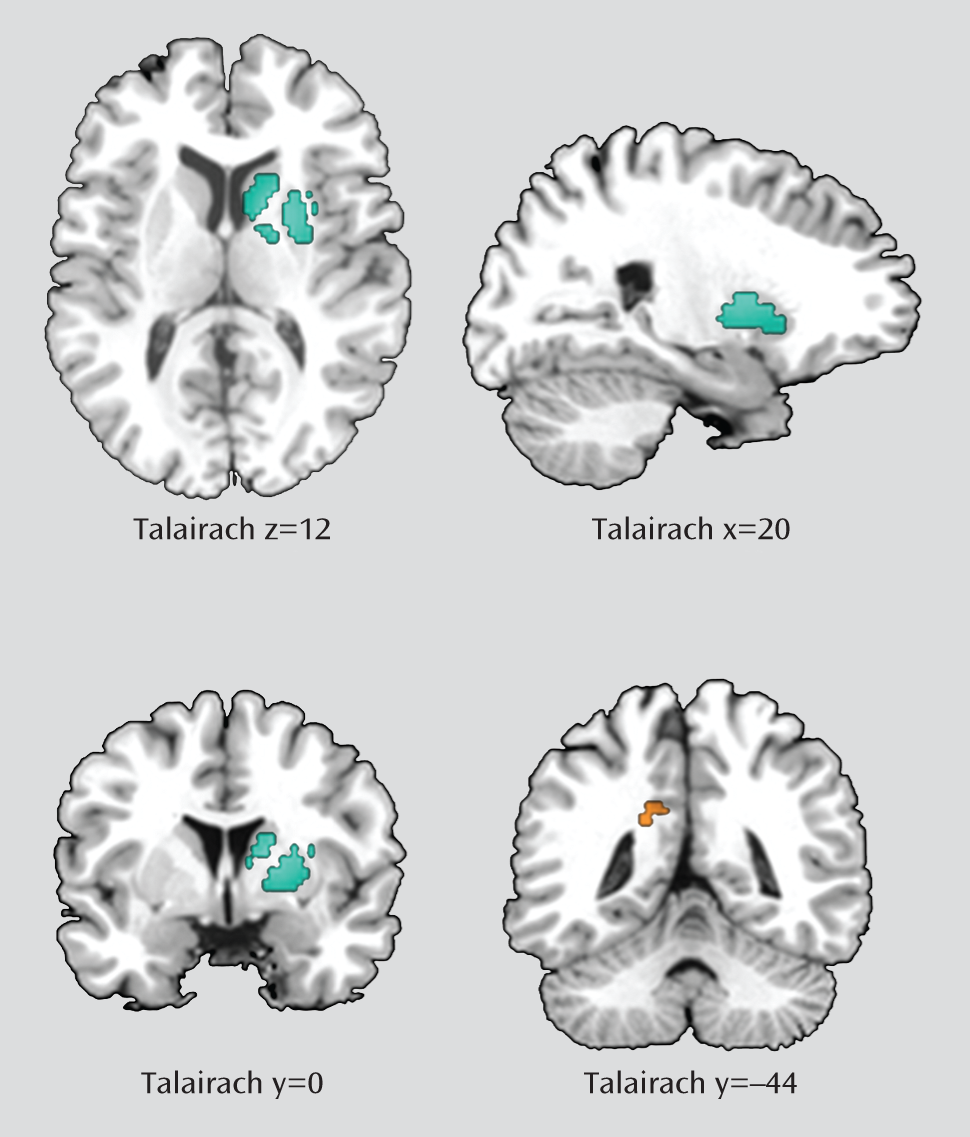
Table 2 and
Figure 1, ADHD patients had significantly smaller gray matter volumes in a large cluster with its peak in the right lentiform nucleus (Talairach coordinates: x=20, y=0, z=12; SDM value=–0.288, p<0.001; 580 voxels). This large cluster also included the caudate nucleus (230 voxels). ADHD patients also had larger gray matter volumes in the left posterior cingulate cortex/precuneus (x=–18, y=–44, z=26; SDM value=0.079, p<0.001; 35 voxels).
Reliability Analyses
A whole-brain jackknife sensitivity analysis, which systematically excluded one study at a time, showed that the results in the lentiform nucleus were highly replicable, as this finding was preserved throughout all of the 14 combinations of studies. The greater volumes in the left posterior cingulate cortex/precuneus remained significant in all but two combinations of studies (
Table 3).
The descriptive analyses of quartiles revealed that the smaller volume in right lentiform nucleus was detected in the median analysis, indicating that at least 50% of the included studies found a significantly smaller volume of gray matter in this region. The left posterior cingulate cortex/precuneus cluster was not detected in this analysis, indicating that less than 25% of studies found significantly larger volumes in this region.
Finally, the subgroup analysis revealed that the above findings remained unchanged when only the nine pediatric studies or only the 11 studies conducting a modulation step were analyzed. Conversely, separate analysis of the five adult studies revealed no significant differences between patients and comparison subjects (
Table 3). Thus, these findings were probably driven by the pediatric studies.
Metaregression Analyses: Effects of Age and Stimulant Medication
Information on both age and medication was available for all 14 data sets, including 378 patients. Among these studies, 207 patients (55%) were receiving medication (methylphenidate, N=182; d-amphetamine, N=1; unidentified stimulants, N=24) at the time of their participation in the studies. The multiple metaregression analysis (
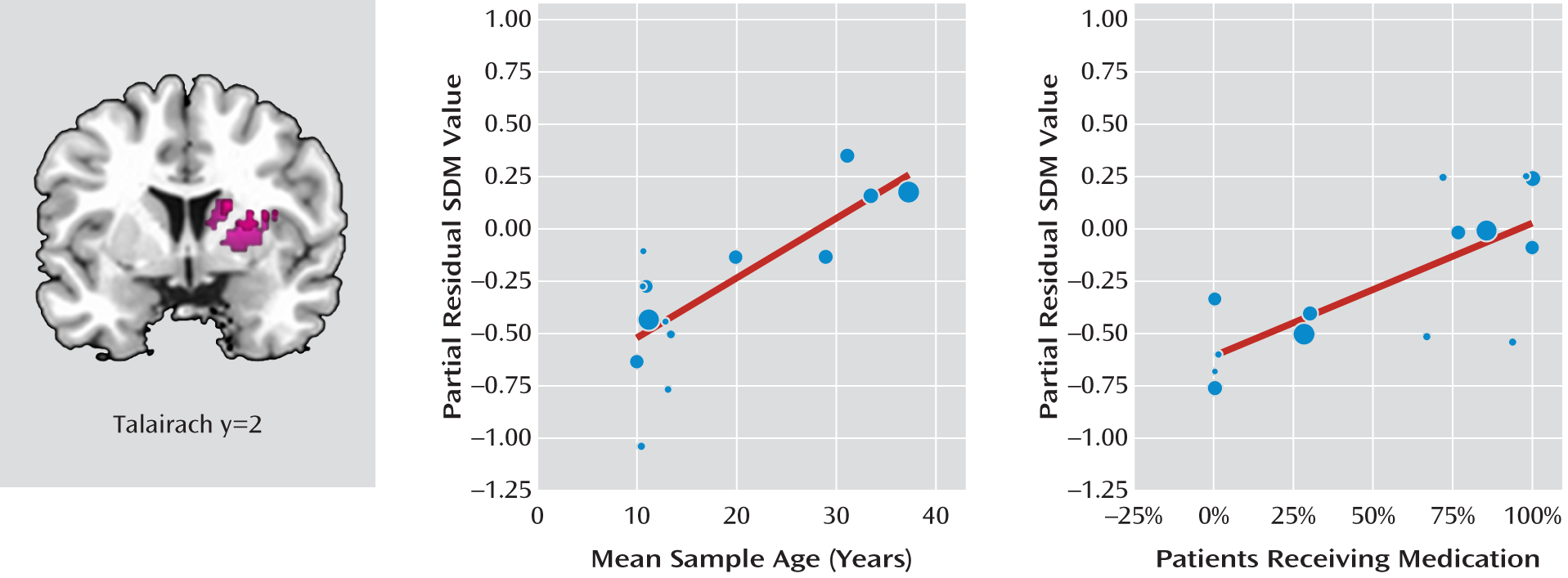
33) revealed that both the mean age and the percentage of patients receiving stimulant medication were independent predictors of increasing (i.e., more normal) gray matter volume in the right basal ganglia (
Figure 2). That is, age was positively and significantly correlated with gray matter volume in the right putamen (x=24, y=2, z=16; SDM value=0.778, p≤0.001; 402 voxels), controlling for the effects of stimulant medication. Similarly, the percentage of patients on stimulant medication was correlated with gray matter volume in the right caudate (x=16, y=2, z=20; SDM value=0.632, p≤0.001; 63 voxels), controlling for the effects of age.
A sensitivity analysis showed that these results remained significant in all combinations of studies, although the cluster size varied, ranging from 55 voxels (excluding Wang et al. [
39]) to 487 voxels (excluding Sasayama et al. [
42]) with regard to age and from four voxels (excluding Wang et al. [
39]) to 122 voxels (excluding Overmeyer et al. [
36]) with regard to stimulant medication.
Discussion
We conducted a meta-analysis of voxel-based morphometry studies of gray matter volume in pediatric and adult patients with ADHD and examined the question of whether age and stimulant medication affect gray matter volume. The main findings were that individuals with ADHD, compared with healthy comparison subjects, display significantly and robustly smaller gray matter volumes in the right lentiform nucleus, extending to the caudate, and larger gray matter volumes in the left posterior cingulate cortex. Both increasing age and stimulant medication were found to be independently associated with more normal basal ganglia volumes.
The lentiform nucleus comprises the putamen and the globus pallidus, and its anterior end is continuous with the lower part of the head of the caudate nucleus. The caudate, putamen, and globus pallidus (i.e., the pallidum) are part of discrete, somatotopically distributed fronto-striato-thalamo-cortical circuits that are essential for higher executive functions that are typically impaired in ADHD, such as inhibition, attention, and timing functions (
2,
44,
45). Our meta-analytic findings are consonant with those of previous region-of-interest structural imaging studies in ADHD that have shown significantly smaller total volume or volume of the caudate head alone, either in the left (
4) or the right (
7) hemisphere, or of the pallidum, either in the right (
4) or the left (
46) hemisphere. These results also confirm and extend those of previous meta-analyses of region-of-interest (
3) and voxel-based morphometry studies in pediatric patients (
20).
The notion of deficits in fronto-striatal neural networks in ADHD is further supported by diffusion tensor imaging and functional MRI (fMRI) studies. Diffusion tensor imaging studies have demonstrated that children with ADHD have compromised integrity in white matter tracts that connect frontal and striatal brain regions (
10). fMRI studies have consistently reported lower functioning in the caudate, putamen, and inferior prefrontal regions during disorder-relevant tasks of motor response inhibition (
47,
48), selective and sustained attention (
49–
51), and timing functions (
45) (for a review, see reference
2). Functional connectivity analyses have furthermore shown that the interregional connectivity between striatal areas and other regions of the brain, such as frontal, cerebellar, and parietal areas, is compromised in ADHD during the resting state as well as during cognitive tasks (
10,
52). Our meta-analytic findings of smaller volumes in the right lentiform nucleus thus reinforce the evidence for compromised structural and functional fronto-striatal neural networks in ADHD.
We also observed larger gray matter volumes in the left posterior cingulate cortex in ADHD patients relative to healthy comparison subjects. The posterior cingulate cortex is particularly relevant for the dynamic allocation of visual-spatial attention to saliency (
53) and has been suggested to be part of the neural interface between attention and motivation (
54). It has consistently been found to be underactivated in ADHD patients in response to salient stimuli, such as oddball trials inhibition errors and rewarded and incongruent stimuli (
2,
51,
54). Our finding of larger gray matter volumes in this region is thus consistent with the literature. The interpretation of this finding should be made cautiously, however, as the obtained volumes in this region were rather small, more inconsistently identified in our reliability analyses, and primarily driven by small studies using uncorrected thresholds.
Surprisingly, no differences in gray matter volumes were detected in the prefrontal cortex despite the fact that six of the included studies reported abnormalities in that region. This may represent a potential false negative result. However, the location of these reported clusters was highly heterogeneous, ranging from very ventral (
37) to very dorsal (
43), and some studies found prefrontal abnormalities only when using a region-of-interest approach and not when using whole-brain voxel-based morphometry (
18).
Age Effects
Our subgroup analysis revealed no significant differences between patients and comparison subjects when only adult studies were included. Consistently, the metaregression analysis revealed that increasing age was correlated with progressively increasing (i.e., more normal) gray matter volume in the right putamen, over and above the effects of medication. This may suggest that over time, ADHD patients may at least partially outgrow their basal ganglia deficits. There is evidence from a longitudinal structural imaging study (
22) that relative to healthy children, children with ADHD have parallel growth curves for nearly all brain regions between the ages of 5 and 18 years but a smaller brain volume overall. The only exception was the caudate, where brain abnormalities became negligible by midadolescence, around age 15 (
22). The basal ganglia reach their maximum volume around age 10, after which there is a progressive decrease in gray matter volume between childhood and adulthood (
55). While there are pronounced gray matter changes between childhood and adolescence in most cortical areas, the gray matter changes between adolescence and adulthood appear to be more restricted to frontal and striatal regions (
55), presumably reflecting the late development of fronto-striatal pathways that mediate late-developing higher-level cognitive functions (
44). These findings are parallel to those of diffusion tensor imaging studies that show a progressive increase in white matter tract development in striatal brain regions between late childhood and adulthood (
56). They are also consonant with consistent evidence from functional imaging studies for progressive development in fronto-striatal brain activation between adolescence and adulthood (
57). Given that striatal gray matter progressively decreases with age in normal adolescent development, it is thus possible that ADHD patients eventually “catch up” with their healthy counterparts at some point in late adolescence. A recent cross-sectional diffusion tensor imaging study (
58) showed abnormal associations between age and caudate white matter tract development in ADHD patients relative to healthy comparison subjects, which was interpreted as a developmental delay that may normalizein late adolescence. A potential caveat, however, is the fact that more pediatric (N=9) than adult (N=5) studies were included in our metaregression analysis, which may have biased the findings toward greater power for pediatric studies. Nevertheless, the adult studies were generally better powered than the pediatric studies and in total included only 50 fewer patients than the pediatric studies.
Effects of Stimulant Medication
The metaregression analysis revealed that the percentage of patients on stimulant medication was correlated with increasing (i.e., more normal) gray matter volume in the right caudate, over and above the effects of increasing age. Stimulant medication is known to block dopamine transporters in the basal ganglia, leading to enhanced extracellular levels of striatal dopamine, while in prefrontal regions it enhances both dopamine and noradrenaline (
29). Dopamine is known to modulate the fronto-striatal neural networks of cognition and motivation that are affected in ADHD (
44). fMRI studies have consistently shown that acute doses of methylphenidate enhance and normalize activation in the basal ganglia as well as their functional connectivity with fronto-cortical and cerebellar regions (
2,
45,
50,
52). It is thus possible that stimulant medication “repairs” abnormal basal ganglia structure in youths with ADHD and thus elicits morphological changes as a consequence of a relative improvement in striatal dopamine levels. The possibility of a “normalization” with stimulant medication of brain abnormalities in ADHD would be consonant with individual structural imaging studies that retrospectively compared subgroups of medicated and nonmedicated children with ADHD matched on symptom severity and found more normal brain structure in medicated compared with unmedicated patients in several ADHD-relevant brain regions, including caudate volume and morphology (
26,
27). Other brain regions that were found to be more normal in medicated relative to never-medicated patients were the inferior frontal, premotor, and parietal areas (
23), the anterior cingulate cortex (
25), the cerebellar vermis (
24), and the thalamus (
27). An important caveat in these studies, however, is the retrospective analysis of relatively small, even if well-matched, patient subgroups, which is confounded by selection bias (with fewer than 20 participants in the majority of studies). Our metaregression analysis extends this prior evidence by including a relatively large number of ADHD patients (N=378) and hence is thus far the statistically most powerful indication that treatment with stimulants may be associated with more normal striatal brain structure. Nevertheless, this important question of a potential normalization of brain structure with stimulant medication can be adequately addressed only in a prospective longitudinal imaging study within a randomized controlled trial.
Relation to Other Fronto-Striatal Disorders
The finding of smaller basal ganglia volumes in patients with ADHD is interesting in view of opposite findings in other fronto-striatal disorders, such as obsessive-compulsive disorder (OCD) and Tourette's syndrome, which are characterized by larger gray matter volumes in this region (
32,
33,
59). These findings are parallel to results from studies using positron emission tomography showing enhanced dopamine availability in the basal ganglia in OCD and Tourette's syndrome (
60,
61) but lower striatal dopamine availability in ADHD (
29). Direct fMRI comparisons between ADHD and OCD have furthermore shown lower caudate activation in patients with ADHD but enhanced caudate activation in patients with OCD during salient oddball stimuli, which was also correlated with the respective disorder-relevant symptom severity (
49). The inverse volume findings are supportive of the notion of an orbitofrontal-striatal dysregulation in OCD, where dorsal prefrontal regions have poor control over overactive and hyperdopaminergic basal ganglia (
62,
63), as opposed to underdeveloped and hypodopaminergic fronto-striatal networks in patients with ADHD (
2). Both a dysregulation of fronto-striatal networks (in OCD and Tourette's syndrome) and a developmental dysmaturation of fronto-striatal networks (in ADHD) may thus be associated with poor behavioral inhibition, which is a shared feature of all these disorders.
Limitations
This study has several limitations, some inherent to all meta-analytic approaches. First, peak-based meta-analyses are based on summarized (i.e., coordinates from published studies) rather than raw statistical brain maps, and this approach may result in less accurate results (
64). Second, the different studies included in this meta-analysis used different statistical thresholds. However, while thresholds involving correction for multiple comparisons are usually preferred, the inclusion of studies with more liberal thresholds is still statistically correct. Indeed, SDM preprocessing uses the coordinates of the voxels with the greatest differences to approximately recreate the statistical parametric map, but it does not make assumptions about whether or not these differences were significant. Third, while voxel-wise meta-analytic methods provide excellent control for false positive results, it is more difficult to completely avoid false negative results (
64). Fourth, there are some inherent limitations to the voxel-based morphometry method, such as reduced effectiveness in detecting spatially complex and subtle group differences (
65). Fifth, some of the included studies reported gray matter density rather than volume. Gray matter density might be understood as a type of volume that has not been corrected by the distorting effects of the normalization to the stereotactic space; therefore, its inclusion in the meta-analysis is valid (it is also a “volume”), although it may add a source of noise. Sixth, we were unable to assess whether ADHD symptom severity was associated with any of the reported structural changes because the included studies used a wide range of measures. Finally, the effects of medication could be assessed only by the percentage of patients receiving stimulant medication, rather than with subtler measures, such as current or lifetime stimulant doses.
Conclusions
Taken together, the results from this meta-analysis suggest that patients with ADHD have significantly and robustly smaller right hemispheric gray matter volumes of the basal ganglia, including the putamen, the globus pallidus, and the caudate nucleus, and possibly larger gray matter volumes in the left posterior cingulate cortex/precuneus. These findings integrate previous inconsistencies in the structural imaging literature of ADHD and provide a coherent picture of the most prominent and replicable structural abnormalities in children with ADHD. Furthermore, our metaregression analysis provides suggestive evidence for maturational changes in the brain and the possibility of structural repair by stimulant medication, although longitudinal within-subject studies are needed to confirm this.
Acknowledgments
The authors thank the authors of the included studies for providing additional data and methodological details that were not reported in the original publications.