To our knowledge, this is the first study to systematically investigate sex differences in the subjective, physiological, and neural correlates of both stress-induced and cue-induced craving in cocaine dependence. Given the differential associations between these two types of craving and treatment outcome (
2), the findings have significant clinical implications. A more precise understanding of similarities and differences in the biological correlates of stress-induced and cue-induced craving in cocaine-dependent women and men will help identify targets for treatment development and lead to improved therapies. The inclusion of a recreational-drinking comparison group facilitated the identification of neural correlates of stress- and cue-related craving that are specific to pathological involvement in substance use behaviors. The clinical implications of the identification of sex-related influences on neural activations related to stress- and drug cue-related craving are described below.
Brain Activations
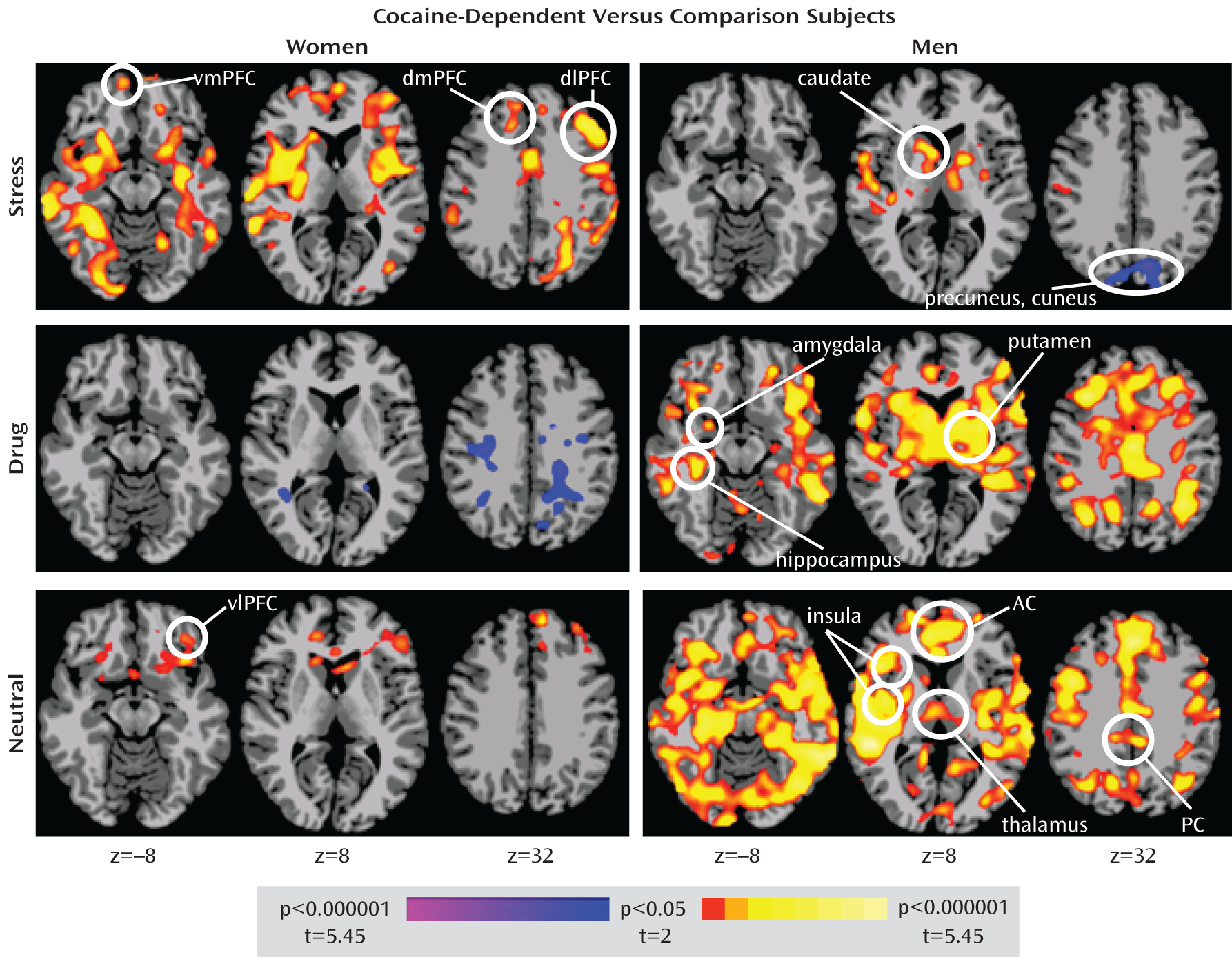
Consistent with our first hypothesis, a three-way interaction of sex, diagnostic group, and script condition involving corticostriatal-limbic circuitry was observed. Our hypothesis that this interaction would involve cocaine-dependent women showing greater corticostriatal-limbic activation relative to comparison women during the stress condition was also supported, although this pattern of increased corticostriatal-limbic activation extended to the neutral-relaxing condition, albeit to a lesser degree. Also consistent with our hypothesis, cocaine-dependent men showed greater corticostriatal-limbic activation relative to comparison men during the substance cue condition. However, in men, cocaine-dependent status was associated to a greater degree with increased corticostriatal-limbic activation across conditions, an effect particularly evident during the drug cue and neutral-relaxing conditions. These differences in brain activation patterns during stress and drug cue conditions in cocaine-dependent men and women cannot be explained by current mood or anxiety ratings, as there were no sex differences in these scores. Thus, these findings suggest that corticostriatal-limbic hyperactivity may be particularly linked to stress cues in cocaine-dependent women and drug cues in cocaine-dependent men, with both cocaine-dependent groups also showing corticostriatal-limbic hyperactivity during neutral-relaxing conditions.
Areas identified as showing overactivation in cocaine-dependent women but not in cocaine-dependent men during the stress condition include the amygdala, hippocampus, insula, anterior cingulate, and ventromedial, ventrolateral, dorsomedial, and dorsolateral prefrontal cortices, regions implicated in emotional regulation, memory function, interoceptive processing, cognitive control, and emotional and motivational processing (
48–
50). Areas identified as being overactive in cocaine-dependent men during the drug condition overlap substantially with those that were overactive in cocaine-dependent women during the stress condition, suggesting that similar neural circuits are responsive to varying degrees in different environmental contexts (stress for women, drug cues for men) for the drug-seeking behavior of cocaine-dependent women and men.
Both similarities and differences were observed in neural responses to stress and drug cues in cocaine-dependent women and men. In men, between-group differences during the stress condition involved predominantly increased activation in the striatum, thalamus, and temporal cortex, regions involved in stress responsiveness, motivation, and auditory processing (
48,
51), and these differences were also seen in women, suggesting an effect of cocaine dependence across the sexes.
Although mainly hyperactivation was observed in cocaine-dependent subjects, hypoactivated regions warrant consideration. During the stress condition, cocaine-dependent men showed relatively diminished activation of the precuneus, a region implicated in attention and impulse control and showing relatively less activation in cocaine abusers during sustained attention (
52,
53). Thus, stress in cocaine-dependent men may interfere with the ability to attend to and control behavior in part through poor recruitment of the precuneus. Analogously, during the substance cue condition, cocaine-dependent women relative to comparison women showed less activation in predominantly dorsal and cortical brain regions, including the inferior parietal lobule, precuneus, and posterior cingulate, suggesting that drug cues in women may interfere with recruitment of attentional processing and impulse control regions.
The relatively increased activation of corticostriatal-limbic circuitry during the neutral-relaxing condition in cocaine-dependent subjects is noteworthy. In cocaine-dependent women, this hyperreactivity involved the ventral striatum, ventromedial prefrontal cortex, lateral orbitofrontal cortex, and inferior frontal gyrus, regions implicated in motivation, reward processing, decision making, and impulse control (
49,
54,
55). These regions were also overactive in cocaine-dependent men during the neutral-relaxing condition, as were the amygdala, hippocampus, insula, and anterior and posterior cingulate cortices, regions implicated in emotional regulation, memory function, interoceptive processing, cognitive control, and emotional and motivational processing (
48–
50). The neutral-relaxing condition served as a nonspecific control condition and is associated with decreases in negative emotion and increases in relaxation and positive emotional responses (
56,
57). In previous studies, cocaine-dependent subjects showed less relaxation and lower positive emotion during neutral-relaxed states relative to comparison subjects (
5), and abstinent, treatment-engaged cocaine-dependent individuals showed higher subjective, behavioral, and physiological responses to stress at baseline and in response to stress, drug cue, and neutral-relaxing imagery exposure (
4,
5,
31,
33). In light of our findings in the present study, this higher baseline distress state in cocaine-dependent subjects appears to be represented as hyperreactivity in the corticostriatal-limbic circuitry during neutral-relaxing imagery.
Correlations With Subjective Craving
Our hypotheses regarding correlations with self-reported craving were partially supported. Consistent with our hypothesis, correlations in men between drug cue-related craving and corticostriatal-limbic activation were observed and involved the hippocampus, insula, and anterior and posterior cingulate, consistent with previous findings (
19,
20,
35,
58). Drug cue-related cravings in cocaine-dependent women also positively correlated with corticostriatal-limbic activation in the hippocampus, insula, orbitofrontal cortex, putamen, and midbrain regions. However, in contrast to our hypothesis, no significant correlations between stress-related brain activations and stress cue-related cravings in cocaine-dependent women survived whole-brain correction, whereas those in men implicated the cerebellum and parietal cortices, regions previously associated with subjective craving, albeit to cocaine cues (
58), and particularly in women (
35). Taken together, the more diffuse correlations between drug cue-related craving and brain activations suggest that subjective drug cue-related craving may be more closely linked to a broader activation of corticostriatal-limbic circuitry than are stress cue-related subjective responses. Furthermore, the similarities between men and women in neural correlates of drug cue-related craving are consistent with preclinical and clinical studies of cocaine self-administration, chronic cocaine exposure, and drug craving that show similarities across sexes in corticostriatal-limbic contributions (
13,
59,
60).
Clinical Implications
Our findings have several clinical implications. First, they suggest that regional brain activation responses during provoked stress cue states in women, drug cue states in men, and neutral-relaxing states in both might serve as neural markers in evaluating the efficacy of new behavioral and pharmacological treatments for cocaine dependence (
8,
61,
62). Direct investigation of this hypothesis (e.g., by using this paradigm in conjunction with clinical trials) is warranted. Second, increased corticostriatal-limbic activity during stress, particularly in cocaine-dependent women, suggests the importance of teaching stress reduction, perhaps with mindfulness techniques (
63,
64), in order to decrease hyperresponsiveness of corticostriatal-limbic regions and restore the brain's ability to discriminate between relevant and irrelevant stimuli and sharpen adaptive and regulatory responses that rely on such information. Given the increased stress cue-related neural activations in cocaine-dependent women in conjunction with an absence of significant neural correlations with subjective responses, techniques to increase patients' ability to utilize contextual cues to identify emotion and improve learning and memory abilities may help during emotional processing and in regulation of emotions and craving states. The observed increased corticostriatal-limbic activity during the drug cue condition in cocaine-dependent men suggests that training to manage exposure or responses to drug cues, through 12-step or cognitive-behavioral approaches, may be particularly helpful for men. The observed increased corticostriatal-limbic activity during the neutral-relaxing state in cocaine-dependent subjects, and particularly men, suggests the importance of exploring methods for decreasing basal corticostriatal-limbic activation, perhaps through mindfulness meditation or exercise.
Strengths, Limitations, and Future Directions
We used a large, well-defined sample to investigate the neural correlates of stress- and drug cue-related craving in cocaine-dependent and comparison subjects. A strength of the study lies in the whole-brain analytic approach used to assess neural activity. Limitations include possible susceptibility artifacts during fMRI and the potential for cues to have lingering subjective or neural effects. Specific design and analytic approaches (e.g., pre-fMRI training in progressive relaxation, relaxation components following each cue, counterbalancing of cue presentation orders, inclusion of baseline neural response as a regressor in analyses) were used to diminish lingering influences. Frequent tobacco smoking was observed among cocaine-dependent subjects. Although imaging findings persisted after correcting for smoking and other differences (e.g., in education level), future studies should examine the potential influence of tobacco use and other individual differences (e.g., in emotional dysregulation) on stress, drug cue, and neutral-relaxing responses. Although there were no sex differences in unprovoked craving measures during early abstinence (days 1 and 4 after admission), future studies should examine subjective and neural correlates of stress- and drug cue-induced craving at different stages of addiction. Although our exploratory analysis did not find a significant influence of menstrual cycle phase in the group and condition effects in women, the sample sizes for women in each phase were small. Future research should examine the influence of sex hormones on the neural correlates of craving. As this study is the first to examine interactive effects of sex and cocaine dependence on neural responses to both stress and drug/alcohol cue exposure, its identification of clinically relevant sex differences has important implications for treatment development.