Adolescence is a period of increased risk-taking behavior, often accompanied by first experiences with alcohol and drug use. This association is not specific for the transition between childhood and adulthood, as increased risk taking, assessed by various measures including the Cambridge Gamble Task (
1), is also observed in substance-abusing adults (
2–
4). Moreover, preference for probabilistic gains has been associated with a greater probability of relapse after abstinence (
5). However, it is still a matter of debate whether increased risk-taking bias is a vulnerability factor or a consequence of chronic substance abuse.
During the use of a broad range of addictive substances, the dopaminergic midbrain-striatal system is recruited (
6). Because this system also plays a crucial role during reward processing, such as in the monetary incentive delay task (
7), it is not surprising that reward processing is aberrant in addiction. Dysfunction in reward-related brain areas (e.g., the ventral striatum) has been reported in addiction in research using positron emission tomography (PET) and functional MRI (fMRI) (
8). As animal models of different stages of addiction have shown, changes in the motivation for drugs and natural rewards are a key component of addiction, with the transition to addiction beginning with changes in the mesolimbic dopamine system (
9). A possible explanation is that individuals who are prone to addiction may seek the more extreme reward of drugs because their experience of conventional rewards is blunted. Drug abusers have marked decreases in dopamine D
2 receptors and in dopamine release, reducing their sensitivity to natural reinforcers (
10). The association between addiction and decreased striatal activation during nondrug reward anticipation (e.g., in the monetary incentive delay task) has also been shown using fMRI (
11,
12).
On the other hand, risk-taking bias as assessed by the Cambridge Gamble Task has been associated in clinical research with insular and ventromedial prefrontal cortex function in patients with mild frontal variant frontotemporal dementia (
13) or lesions of the ventromedial prefrontal cortex and insular (
14,
15). These findings complement reports of activation during different measures of risk taking in functional neuroimaging studies (
16,
17). Most interestingly, though, the striatum plays a role in risk processing as well, as indicated by card gambling and financial decision-making tasks (
18–
20). Moreover, it has been shown that in a risky card game, areas recruited during risk processing overlap with those modulated by reward value within the ventral striatum (
18). Notably, both the ventral striatum and the ventromedial prefrontal cortex are responsive to reward, with activation of the ventromedial prefrontal cortex (e.g., during reward anticipation in the monetary incentive delay task) being modulated by both the value and the probability of reward (
21,
22). Conversely, it has been demonstrated that the insular cortex signals the expectancy of aversive outcomes in learning from punishment and instrumental conditioning (
23,
24). Thus, it is of relevance that the ventral striatum, and specifically the nucleus accumbens, receives convergent input from the insula and the ventromedial prefrontal cortex (
25).
In light of these findings, we suspect that the link between risk-taking behavior and drug addiction may stem from a common neural basis: a relative hypoactivation of the ventral striatum prior to initial substance abuse. Research in rodents and nonhuman primates has shown that general behavioral patterns and reduced activation of the dopaminergic reward system before exposure to a drug mutually predict the level of voluntary drug intake (
26,
27). Rats bred for high impulsivity—a different pattern also associated with drug abuse (
28)—exhibited both a decreased dopamine receptor density in the ventral striatum and very high rates of cocaine self-administration (
26). Such a relationship has not been observed in humans.
In this study, we investigated whether risk-taking bias in adolescents is associated with decreased striatal reward processing and therefore shares the neural correlate of addiction prior to a possible onset of substance abuse. We also explored the structural basis of this potential association.
Method
Participants
Participants were drawn from the IMAGEN study; a detailed description of the sample is available elsewhere (
29). For the present study, 299 adolescents were drawn from the first group of IMAGEN subjects available for data analysis. Adolescents who reported potentially problematic substance use were not included in the final main sample. Indications for problematic substance use were signs of nicotine dependence, defined as a score >0 on the Fagerström Test for Nicotine Dependence (
30), or likelihood of alcohol abuse, defined as a score >7 for males or >6 for females on the Alcohol Use Disorders Identification Test (AUDIT) (
31). Participants were also allocated to the substance use group when they reported having tried hashish or marijuana more than one or two times or having ever tried amphetamines, cocaine, crack, Ecstasy (3,4-methylenedioxymethamphetamine), GHB (γ-hydroxybutyric acid), glue (sniffing), heroin, LSD, ketamine, or psychoactive mushrooms. By these criteria, 33 participants were categorized as having potentially problematic substance use, leaving a main sample of 266 participants. The characteristics of the main sample are summarized in
Table 1.
As expected, the large majority of the main sample reported having tried alcohol or cigarettes at some time, and excluding these participants might have led to a nonrepresentative sample. However, to rule out causal influences of substance consumption on our results, we conducted a control analysis including only participants without lifetime substance use except for trying an alcoholic beverage on one or two occasions at the most (e.g., a sip from a parent's drink); 88 participants were in this group, 48 of them female.
Additionally, we compared the potentially problematic substance use subgroup to a comparison group drawn from our main sample. From the 33 participants in the substance use group, two had to be excluded to keep handedness equal between groups. Thus, we compared 31 adolescents with potentially problematic substance use and 31 comparison adolescents from our main sample. The groups were matched for gender, intelligence estimate (sum score of the block design, matrix reasoning, vocabulary, digit span, and similarities subtests of the WISC-IV [
32]), pubertal status (based on a modified version of the pubertal development scale [
33]), and handedness (
Table 2). We chose to match for pubertal status instead of age because adolescents of the same age vary in their stage of development.
After adolescents and their parents received a complete description of the study, we obtained written informed consent from the parents and assent from the participants.
Risk Taking Assessment
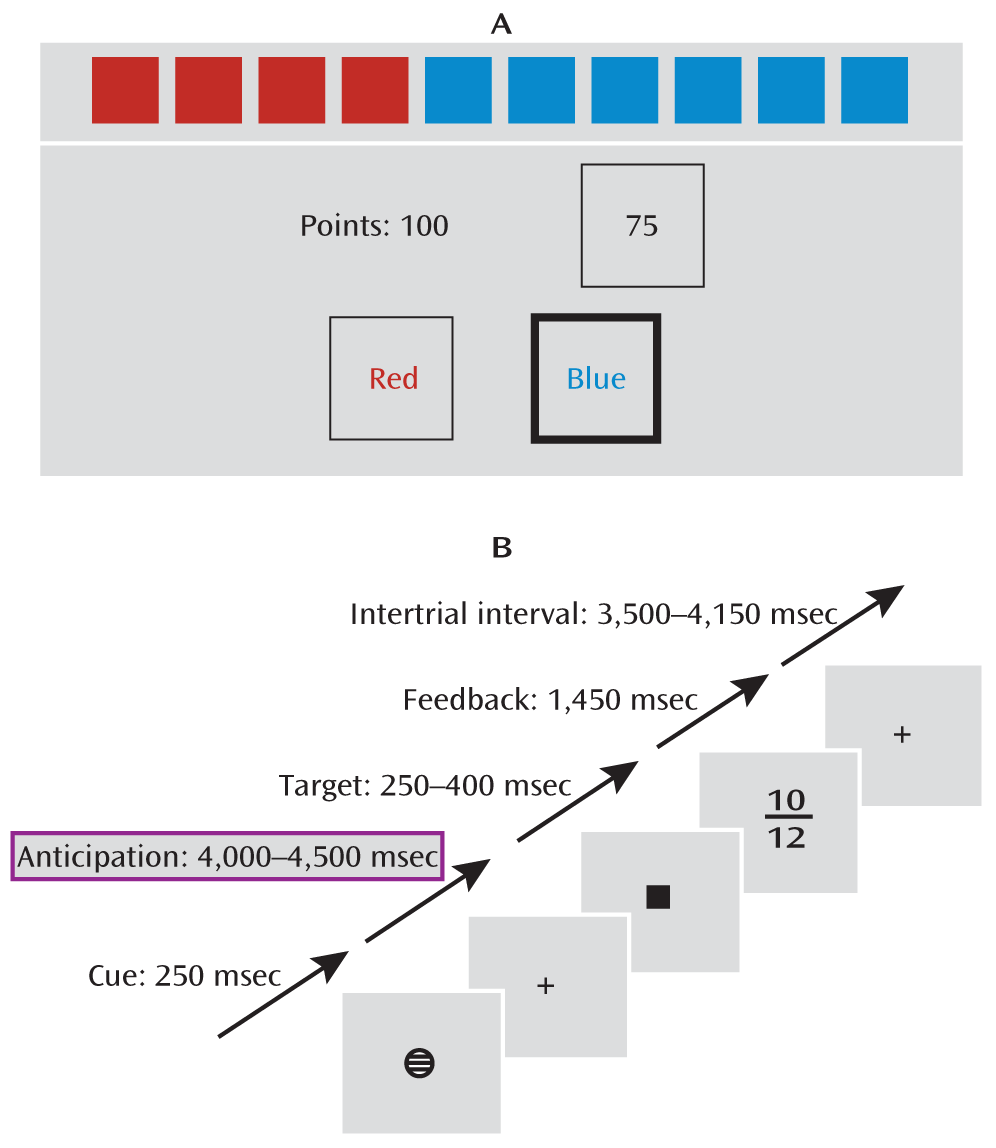
To assess the participants' risk-taking bias, we asked them to complete the Cambridge Gamble Task (
1,
34) (
Figure 1). This task has been shown to discriminate addicted individuals from healthy comparison subjects on the basis of their risk-taking behavior (
3). During the task, participants were presented with a row of 10 red or blue boxes on a computer touchscreen. They were instructed to guess whether the target was hidden behind a red or a blue box. After placing their guess, they were presented a range of points, in either ascending or descending order, that they could select to bet on their guess. If they had picked the right color, they gained the number of points they had bet, and otherwise they lost it. The measure of risk-taking bias was the average percentage of points bet given that the participant had chosen the more likely option.
Reward Task
To assess reward processing during fMRI, a modified version of the monetary incentive delay task (
7;
Figure 1) was used. Each trial consisted of anticipation, response, and feedback. Before the anticipation phase, a cue signaled the position of the target as well as the type of reward that could be attained by a correct response. Different cues distinguished between large (10 points), small (2 points), and no (zero points) rewards. After a random anticipation interval of 4,000–4,500 msec, the target appeared. Participants were instructed to respond to the target as quickly as possible via button press and were informed that the points earned would be converted into chocolate treats. The duration of the target appearance was adapted to the performance of the subject (250–400 msec) such that performance would be successful (reaction within the interval that the target was shown on screen) on approximately 66% of all trials. Immediately after the response, feedback indicated the points earned in that trial as well as the total points earned during the task. Large, small, and no win conditions were randomized throughout the task (22 trials each, summing up to 66 trials total).
Imaging Parameters
All MRI data were acquired using 3-T MRI scanners made by several manufacturers (Siemens, Philips, General Electric, and Bruker) in the eight European IMAGEN assessment sites. All sites used the same scanning parameters (
29). In the functional task, 300 volumes per participant were obtained, each comprising 40 slices. The slices were aligned to the anterior commissure-posterior commissure line (2.4-mm thickness, 1-mm gap, TR=2.20 seconds, TE=30 msec). The scanning procedure required 11 minutes. High-resolution anatomical MR images were acquired, including a three-dimensional T1-weighted magnetization prepared gradient echo sequence (MPRAGE) based on the Alzheimer's Disease Neuroimaging Initiative protocol. The structural image comprised 160 slices (1.1-mm thickness, TR=2,300 msec, TE=2.8 msec) and required 9 minutes for acquisition.
Functional and Structural MRI Preprocessing and Analysis
All image analysis procedures were conducted using SPM8 (Wellcome Department of Cognitive Neurology, Institute of Neurology, University College London). For the functional analysis, echo planar images were coregistered with the T1 structural image, then realigned and resliced. A first-level model was constructed on the unsmoothed single-subject data using each of the following regressors twice (for successful and unsuccessful trials): anticipation large reward, anticipation small reward, anticipation no reward, feedback large reward, feedback small reward, feedback no reward. Trials in which participants failed to respond were modeled separately. Individual movement parameters were included as covariates. In the present study, we focused on the anticipation of large reward (compared to baseline, not to another condition, ensuring that we did not subtract any relevant activation). Using the DARTEL toolbox as implemented in SPM8, we created a custom template from the participants' T1 images and normalized single-subject contrast images to Montreal Neurological Institute (MNI) space, then smoothed with a 6-mm Gaussian isotropic kernel.
In voxel-based morphometry (
35), spatially normalized structural scans are compared on a voxel-by-voxel basis. However, instead of directly comparing MR image intensity, structural scans are segmented into gray and white matter, and then, after smoothing, the gray matter partition is subjected to a voxel-by-voxel statistical test. This test can be either a comparison of two groups or a correlation analysis, testing whether a variable is correlated with structural variability in gray matter. In the present analysis, T
1 images were segmented using the SPM8 segmentation toolbox, then modulated and normalized to MNI space using DARTEL. These images were smoothed with a 4-mm Gaussian isotropic kernel.
Because of gender differences in risk taking, a two-sample t test (males compared with females) was conducted for both functional and structural data. Risk-taking bias was modeled individually for each gender, which resulted in modeling the association with risk-taking bias while controlling for the gender difference in this measure. In the comparison between the substance use group and the comparison group, only assessment site was included as a covariate of no interest as the groups were matched on other relevant measures. In all other analyses, handedness, pubertal status (
33), an intelligence estimate (
32), and assessment site were included as covariates of no interest. In the voxel-based morphometry analysis, total gray matter volume was also included. As pubertal status and gray matter volume differed between males and females, we also modeled these covariates individually for each gender. For the main analyses, the threshold for significance was set to an alpha of 0.05, family-wise error corrected, using small-volume correction unless otherwise indicated. Correction for the ventral striatum was based on an 18-mm diameter sphere centered at coordinates x=14, y=8, z=–8, as indicated in previous studies (
22,
36).
To clarify the relation between structural and functional findings, the peak voxels identified in the main analyses were, separately for each hemisphere, incorporated into a mediation analysis. This analysis tests whether the covariance between two variables (i.e., the source and the outcome) can be explained by a third variable (the mediator). A significant mediator is one whose inclusion as an intermediate variable significantly affects the slope of the x-y relationship. Suspecting that the association between risk-taking bias and lower striatal activation might be triggered by lower structural integrity of the striatal region, we defined the peak voxel in the functional analysis as the source and the peak voxel in the structural analysis as the mediator, for both the right and the left striatum, with risk-taking bias as outcome. The mediation analysis was carried out using MATLAB code by T.D. Wager (
http://wagerlab.colorado.edu/), based on a standard three-variable path model (
37) with an accelerated bias-corrected bootstrap test for statistical significance (
38). We kept a significance threshold of 0.05 for all relevant paths: the indirect path a (relation source–mediator), the indirect path b (relation mediator–outcome, fixed for the source), and the mediation effect ab (the product of a and b, also defined as the reduction of the total relationship between source and outcome (total relationship c) by including the mediator into the model (direct path c′).
Figures are displayed with a threshold of p<0.001, uncorrected, with an extent threshold of five contiguous voxels, and projected onto the mean structural scan of all volunteers who were used for the construction of the DARTEL template.
Results
As expected, the ventral striatum was strongly activated bilaterally during the anticipation of large rewards (x=14, y=8, z=–3; t=17.19, df=254, p<0.00001, family-wise error corrected; and x=–16, y=9, z=–3; t=19.26, df=254, p<0.00001, family-wise error corrected). However, the peak activation during anticipation of large rewards was located in the insula bilaterally (x=33, y=21, z=7; t=23.72, df=254, p<0.00001; and x=–30, y=21, z=7; t=26.08, df=254, p<0.00001; p values whole brain family-wise error corrected).
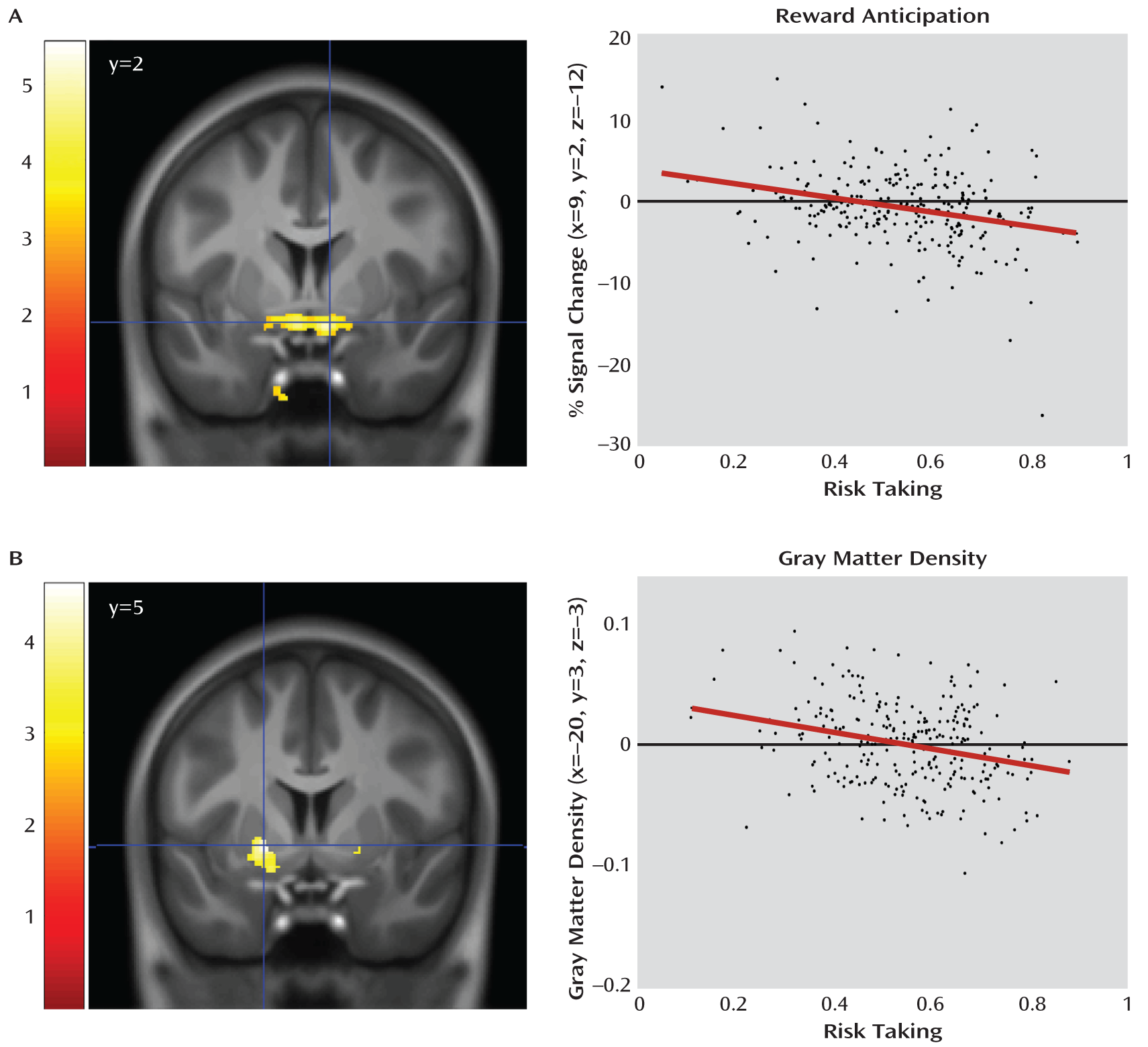
More importantly, and in agreement with our hypothesis, the magnitude of the striatal activation was inversely correlated with individual risk-taking bias (x=9, y=2, z=–12; t=4.73, df=252, p=0.0003; and x=–8, y=3, z=–12; t=4.30, df=252, p=0.002; p values family-wise error corrected;
Figure 2), an association of medium effect size (d=0.57 and d=0.52, respectively). To further explore the structural basis of this association, we used voxel-based morphometry to correlate risk taking with gray matter density in the striatum, again finding a bilateral and inverse association (x=20, y=5, z=–5; t=3.67, df=250, p=0.019; and x=–20, y=3, z=–3; t=4.61, df=250, p=0.0007; p values family-wise error corrected;
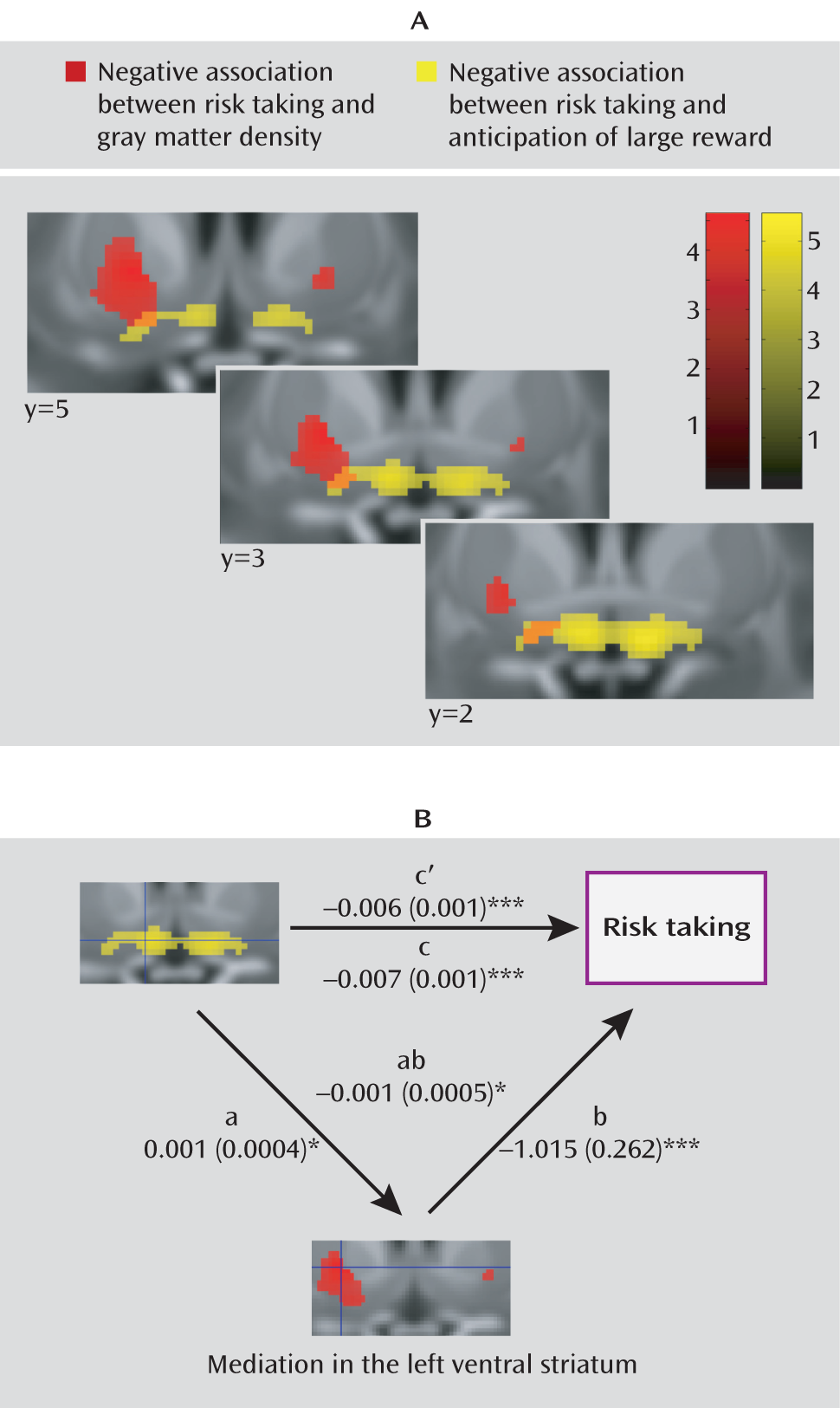
Figure 2). This association was of medium effect size as well (d=0.44 and d=0.57). Attempting to disentangle the relationship between our functional and structural findings, we conducted a mediation analysis for the right and the left striatum separately, testing whether the peak voxels identified in the structural analysis mediated the peak associations with risk-taking bias in the functional analysis. In the left striatum, the association of the left striatal activation with risk-taking bias (
Figure 3) was partially mediated by gray matter density (path coefficients, a=0.001 [SE=0.0004], p=0.028; b=–1.015 [SE=0.0262], p=0.0002; ab=–0.007 [SE=0.01], p=0.023). However, both gray matter density and activation during reward anticipation in the left striatum independently contributed to the explanation of risk-taking bias when the other variable was fixed (b=–1.015 [SE=0.0262], p=0.0002; c′=–0.006 [SE=0.001], p=0.0002). Such an effect was not observed in the right striatum (a=0.0007 [SE=0.0004] p=0.134; b=–0.851 [SE=0.243], p=0.001; ab=–0.0006 [SE=0.0004], p=0.156).
Additionally, we conducted a control analysis in 88 participants with virtually no lifetime substance use (see the Method section). Even in this subsample, the negative association between reward anticipation and risk taking was significant in the right ventral striatum (x=8, y=2, z=–11; t=3.58, df=74, p=0.028, family-wise error corrected), with a large effect size (d=0.80); in the left ventral striatum, the association fell short of corrected significance (x=–18, y=12, z=–14; t=2.52, df=74, p=0.007, uncorrected), although it had a medium effect size (d=0.58).
We also compared the group of adolescents with potentially problematic substance use who were not included in our main analyses (N=31) with a matched comparison group (N=31) drawn from the main sample. In line with our predictions, the substance use group took more risks as assessed by the Cambridge Gamble Task (p=0.036;
Table 2). In addition, we found significantly less activation in the substance use group in the left ventral striatum (x=–9, y=12, z=–14; t=3.52, df=55, p=0.038, family-wise error corrected). The corresponding activation in the right striatum did not reach corrected significance (x=12, y=9, z=–2; t=2.97, df=55, p=0.002, uncorrected).
Discussion
Results from our large data set show that risk-taking bias, an established correlate of addiction (
2–
4), is associated with decreased activation during reward anticipation and with structural changes of the brain's core reward signaling system in healthy adolescents. Jointly, our functional and structural findings provide evidence that risk-taking bias is associated with a pattern of the reward system that has previously been linked to substance abuse in humans (
8,
10,
11) and vulnerability to addiction in rodents and primates (
10,
26,
27). Thus, it extends research indicating that general behavioral patterns such as risk taking or impulsivity can predict drug-seeking behavior, as shown in high-impulsive rats for cocaine self-administration (
26) or in humans for different measures of impulsivity and substance use (
28). However, while risk-taking bias might partially overlap with impulsivity, these two patterns seem to be distinct traits, both connected to substance abuse (
2–
4,
28). Whereas our study leaves open the question of how such patterns are attained, previous findings suggest that they may be inherited (
26) or influenced by environmental factors, including social rank (
27).
Risk-taking bias was assessed not by a questionnaire, which might have been influenced by social desirability, but by a well-validated neurobehavioral test. The monetary incentive delay task, on the other hand, is established as a standard instrument to assess reward processing. The large activation of the ventral striatum in the main effect of reward anticipation confirms the task's validity for our research question. Conversely, the observed association cannot be a result of a “generally decreased activation” in more risk-taking individuals, as reward anticipation activated the whole reward network (maximally the insula), while risk-taking bias was associated only with striatal activation. Thus, the neural association between risk taking and reward anticipation is highly specific to the ventral striatum.
The risk taking-related decrease in activation is confined to the most ventral and medial portion of the striatum, presumably the nucleus accumbens. This is consistent with the observation that the nucleus accumbens receives dense projections from the insula and the ventromedial prefrontal cortex, areas essential for normal risk-taking behavior (
25). The structural analogue of this association extends more laterally into the ventral putamen, partially mediating the functional association in the left striatum. In light of this very limited mediation effect, we consider decreased striatal activation during reward anticipation and gray matter density as separable though weakly connected explanatory factors of risk-taking bias. This notion is compatible with the observation of a recursive modulation of the reward circuit through striato-nigro-striatal loops, as proposed by Haber and Knutson (
25).
Adolescents with potentially problematic substance use were excluded from the association analyses performed in the main sample, and the reported activation decrease also holds in a subgroup of participants who practically never used alcohol, cigarettes, or drugs. Conversely, when compared with matched comparison subjects, the group of adolescents with potentially problematic substance use showed stronger risk-taking bias as well as decreased activation of the ventral striatum. The substance use group was from the same population as our main sample, which strengthens support for the interpretation that these properties may represent a common link to a predisposition to problematic substance use. This subsample represents a group highly susceptible to substance abuse, directly emphasizing the relevance of our findings.
Nevertheless, our study has several limitations. First, because the analysis is confined to cross-sectional data, no definite conclusions can be drawn about a causal relationship with future substance use. However, the literature clearly shows that both risk-taking behavior (
2–
4) and dysfunction of the striatal reward system (
8,
10,
11,
26,
27) are linked to addiction. Moreover, our results in the substance use group imply that these factors have a role in addiction. A reduced follow-up investigation of the IMAGEN sample (including mostly questionnaires) will be conducted 2 years after initial assessment, and another, more informative follow-up study planned for when participants have reached young adulthood is expected to provide a more definite outcome. Another potential limitation is that although our control analysis in virtually substance-naive adolescents shows that substance use is not the cause of the reported association, we cannot exclude the possibility that substance use may still have influenced our results in some way. Given the large sample size, we could not assess cotinine levels or urine toxicology, which could have improved the reliability of the substance use reports. Another point to bear in mind is that our results apply to adolescents and cannot be generalized to adult samples. Finally, the data collection at different sites with different MRI scanners may have added some variance to our MRI data. However, we accounted for this variance in all analyses.
In summary, our data show a strong relationship between risk taking and functional and structural properties of the reward system in nonaddicted adolescents. These results offer a neural explanation for how risk-taking bias may contribute to a predisposition for addiction. Thus, our study makes risk taking a likely candidate in the current search for vulnerability factors for drug abuse.