Delirium is described as an acute decline in consciousness and cognition associated with impaired attention. Although delirium is a common and potentially life-threatening clinical syndrome (
1), little is known about the specific mechanisms underlying the disorder. Previous neuroimaging studies have suggested that general brain abnormalities, such as cortical atrophy, ventricular enlargement, and increased white matter lesions, may predispose individuals to developing delirium (
2). Resting-state functional MRI (fMRI) is a promising technique for reflecting spontaneous neuronal activity and connectivity (
3). This approach has received more attention among researchers examining cognitively compromised patients (
4) and disorders of consciousness (
5), as it requires no stimulus-response sequence and is highly replicable (
6). Thus, resting-state fMRI may be useful in investigating neural networks in delirium.
When applying this method in studying delirium, two specific neural systems should be taken into account. The first is the default-mode network, which is a group of brain regions showing greater levels of activity at rest than during attention-based tasks. These regions include the posteromedial/anteromedial cortices and temporoparietal junctions (
3,
7). Among patients in vegetative or comatose states, connectivity of the default-mode network has been shown to be decreased in proportion to the degree of consciousness impairment (
5). Abnormal default-mode network activity has been also observed in patients with Alzheimer's disease (
8). In particular, decreased posteromedial default-mode network activity has been associated with cognitive impairment (
9). The posteromedial cortex, including the posterior cingulate cortex, is a central node of the default-mode network (
10–
12), and it is a frequent focus of examination in studies of cognition, attention, and consciousness (
13). Given that impaired consciousness and cognition are the primary symptoms of delirium, the posterior cingulate cortex and default-mode network are likely sources of core dysfunctional activity in patients with delirium.
The second system that should be taken into account includes the subcortical regional connections, which are associated with acetylcholine and dopamine. One leading hypothesis regarding the pathogenesis of delirium points to neurochemical abnormalities (
14). The role of cholinergic deficiencies has received the greatest amount of attention, as this neurotransmitter system is involved in sleep, attention, arousal, and memory (
15,
16). Dopamine excess may also be involved, as it has a regulatory influence over the release of acetylcholine (
16,
17). Accordingly, the strength of resting-state functional connectivity between regions producing or utilizing acetylcholine and dopamine may be an appropriate target for elucidation of the pathophysiology of delirium. Based on the hypothesis proposed by Gaudreau and Gagnon (
17), which emphasized the role of the thalamus as well as interactions between neurons related to acetylcholine and dopamine in delirium, multiple subcortical regions (including the intralaminar thalamic nuclei, the mesencephalic tegmentum, the nucleus basalis of Meynert, the ventral tegmental area, the caudate, and the putamen) are appropriate regions of interest to target.
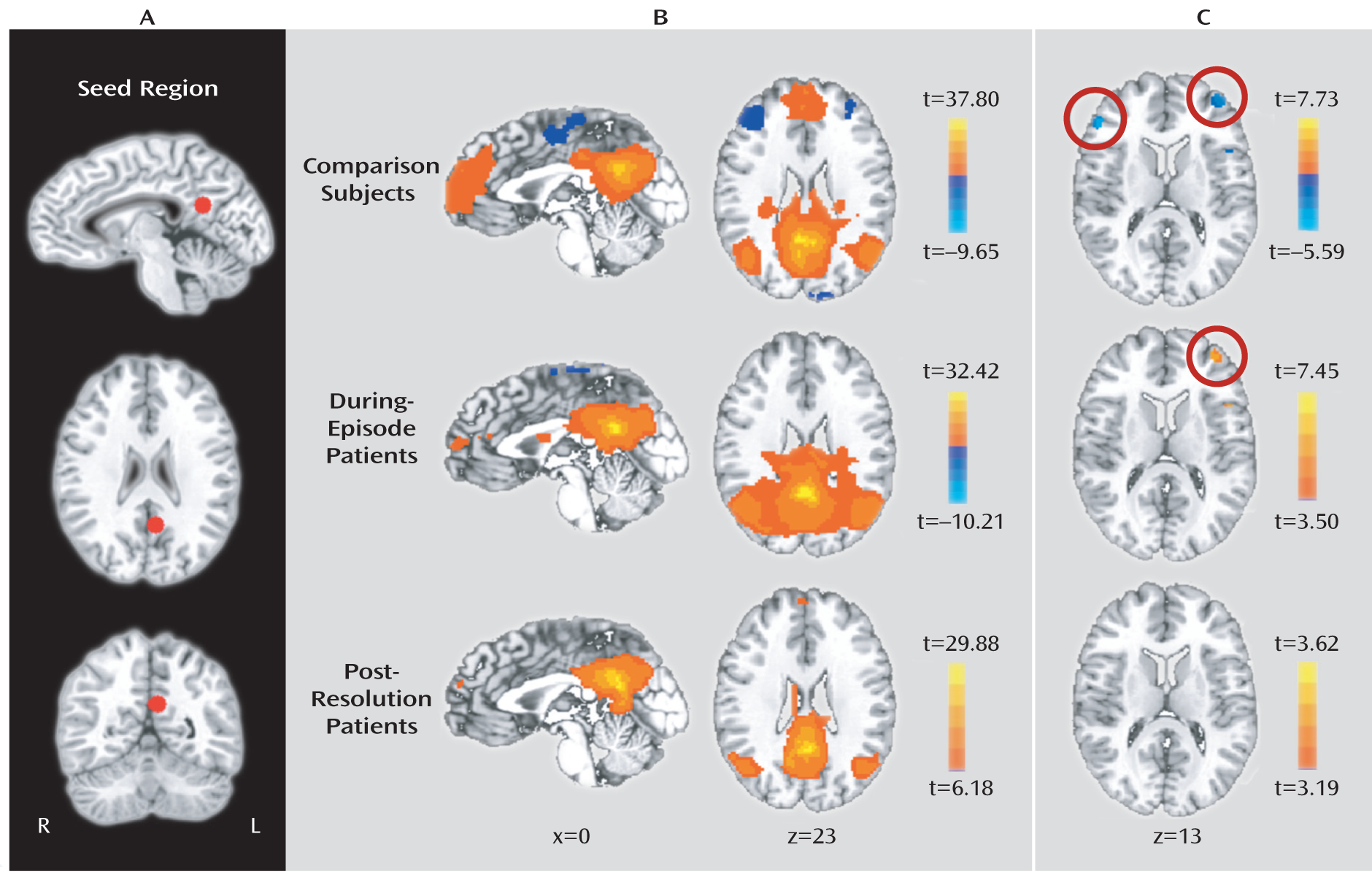
This study was designed to serially investigate resting-state brain networks during and after an episode of delirium using fMRI. We hypothesized that cortical default-mode network connectivity and subcortical neurotransmitter-related functional connections would be disrupted during an episode of delirium and normalized after the resolution. In order to verify these hypotheses, we examined default-mode network connectivity using the posterior cingulate cortex as a seed region, and we measured functional connectivity strengths among subcortical regions associated with the acetylcholine and dopamine pathways.
Discussion
To explore delirium-specific neural dysfunctions, we serially scanned patients during and after an episode of delirium using resting-state fMRI and analyzed functional connectivity among the brain regions. The between-group comparison revealed that posterior cingulate cortex activity was more strongly associated with dorsolateral prefrontal cortex activity in during-episode patients relative to those of postresolution patients and comparison subjects. These findings are in line with the results between the posterior cingulate cortex and the dorsolateral prefrontal cortex from the one-sample group analysis, which showed a negative correlation in comparison subjects and a positive correlation in during-episode patients. Our finding of the inverse correlations between the two regions in comparison subjects has been also demonstrated in previous studies for healthy subjects (
7,
10,
11), suggesting their reciprocal relationship during a resting state.
Functional connectivity studies have revealed some resting-state networks, including the default-mode network and executive network (
29). Here, the posterior cingulate cortex is a hub node of the default-mode network, whereas the dorsolateral prefrontal cortex is a part of the executive network or task-positive component (
10,
30). The reciprocal relationship of the two regions may contribute to individuals' preparation for or adaptation to unexpected or novel environmental events (
10,
11). Therefore, reduced anticorrelation or positive correlation between them in during-episode patients may suggest a reversal of the relationship between the two reciprocal networks during an episode of delirium, and this abnormal interaction may contribute to some clinical features, such as reduced clarity of awareness of the environment. Anticorrelation is an inverse correlation between intrinsic fluctuations in neuronal activity of the seed region and those of the target region in brain functional networks. In addition, the anticorrelation strength between these two neural systems has been associated with behavioral consistency in performing cognitive tasks (
31), which is a useful index for an efficient regulation of attention (
32). Reduced anticorrelation between the two regions was revealed in patients with attention deficit hyperactivity disorder, and this reduction was associated with attentional lapses (
33). A resting-state anticorrelation between default-mode network and frontolateral cortex activities was presented during wakefulness but was decreased in proportion to propofol-induced loss of consciousness (
34) and was reduced in the vegetative state (
35). Taken together, a disruptive alteration in the reciprocity between the posterior cingulate cortex and the dorsolateral prefrontal cortex may explain impaired attention or consciousness, which is considered a cardinal symptom of delirium (
36).
It deserves mention that this anticorrelation was not revived after the recovery of delirium, as shown in
Figure 1. This means that delirium-related default-mode network changes can be maintained for a certain period even after clinical improvement. The interval of 5.8 days between the scans in this study was longer than a reported average duration of 3.4 days of delirium in an intensive care unit (
37), suggesting that the time gap was enough to achieve an improvement. In addition, scores on the Memorial Delirium Assessment Scale and the Delirium Rating Scale–Revised-98 were sufficiently low in postresolution patients. However, given that some symptoms, including inattention, may persist long after the resolution of delirium and result in cognitive impairment (
38), our postresolution patients could also have subclinical residual symptoms. Therefore, the persistence of reduced anticorrelation between the posterior cingulate cortex and the dorsolateral prefrontal cortex may underlie some residual delirium symptoms such as mild inattention. Alternatively, considering that there were no significant correlations between the degrees of regional changes in functional connectivity strengths and changes in the delirium severity measures, this remaining effect may reflect only a delayed recovery of the disruptive connectivity.
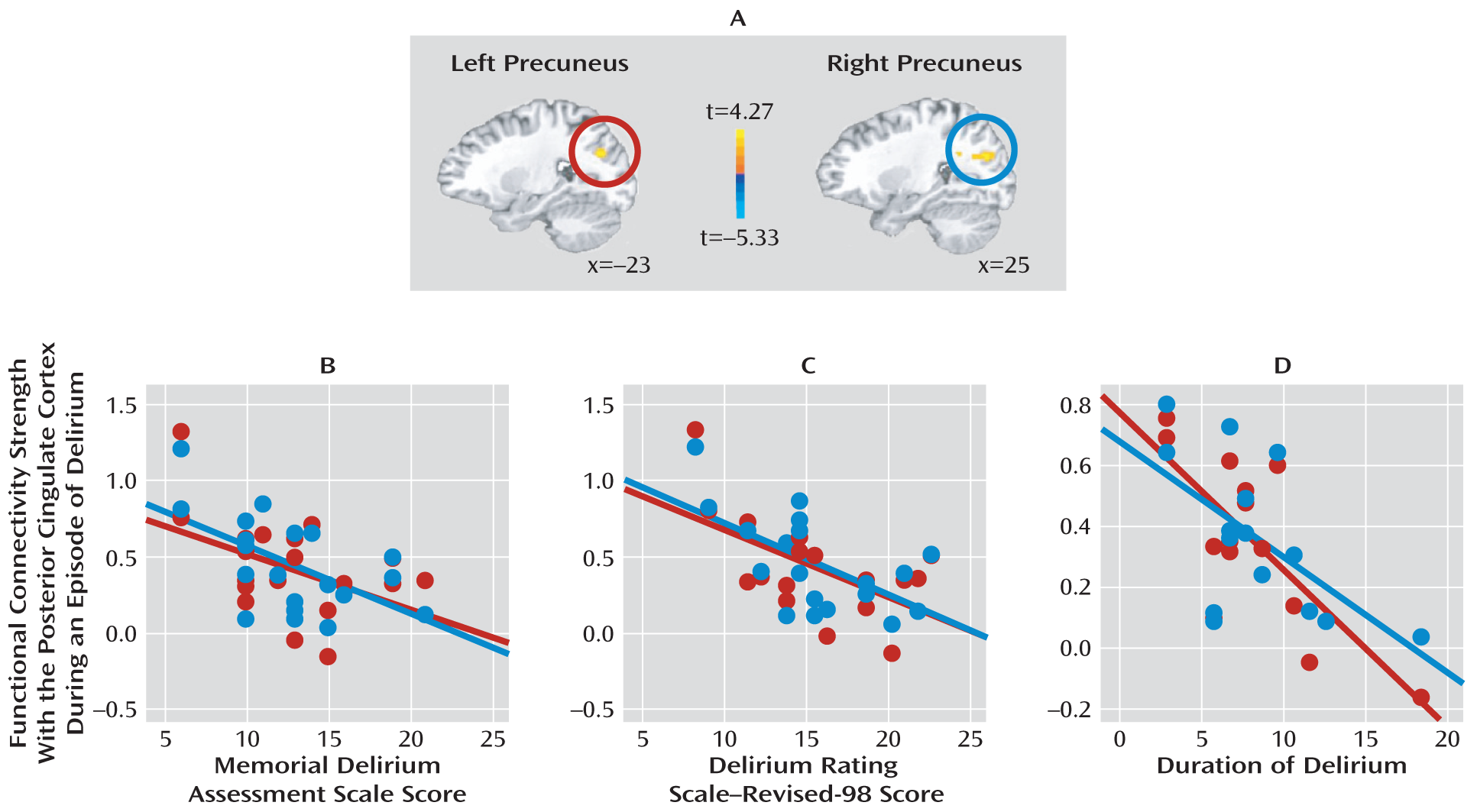
This study also revealed a strong correlation between precuneus and posterior cingulate cortex activities during an episode of delirium, suggesting enhanced integration of the posteromedial default-mode network. This was in contrast to decreased functional connectivity between these two regions in dementia patients (
8,
12), suggesting different roles of the posteromedial default-mode network between delirium and dementia. As shown in the results, more increased functional connectivity between these two regions was associated with lower scores on the Memorial Delirium Assessment Scale and the Delirium Rating Scale–Revised-98, suggesting that enhanced integration in the posteromedial default-mode network during an episode of delirium may help to prevent exacerbation of delirious symptoms. In addition, posteromedial default-mode network connectivity was inversely correlated with duration of delirium, suggesting that the enhancement may help to facilitate the resolution. Fluctuating course, including repeated periods of temporary clear consciousness, is one of the diagnostic features of delirium (
39), and this might be attributed to an action of certain protective factors that help patients restore normality. Therefore, enhanced integration in the posteromedial default-mode network may account for the fluctuating course of delirium.
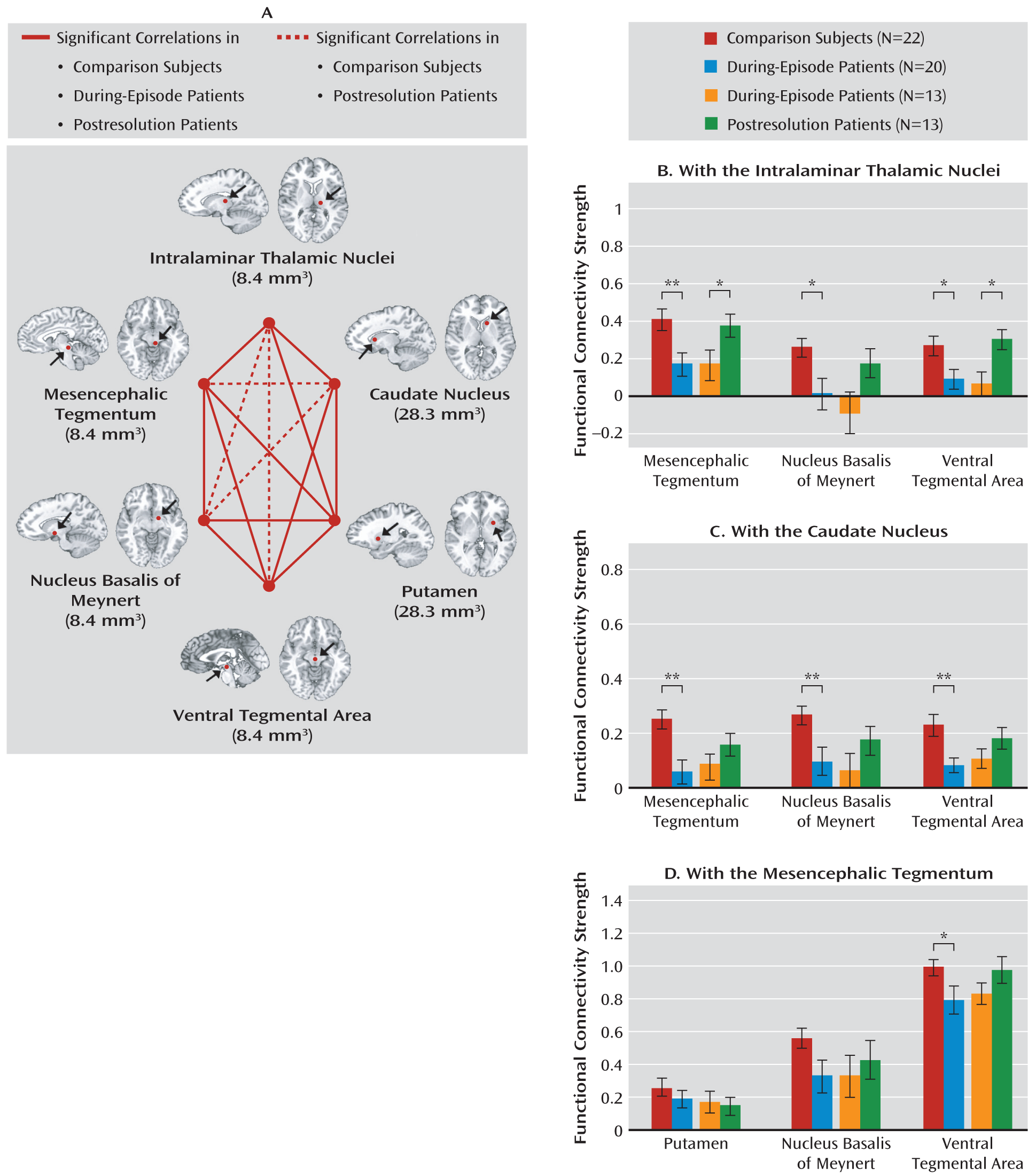
As Laureys mentioned (
40), awareness may not be exclusively related to the frontoparietal network, and the relationship between the global levels of brain functioning and states of awareness may not be absolute. As suggested by Gaudreau and Gagnon (
17), critical substrates of delirium may be located in the striatum, the ventral tegmental area, or the thalamus. Our subcortical analysis showed that the intralaminar thalamic nuclei and caudate appeared to have a notable influence on functional disconnections during an episode of delirium. When examining connectivity with the intralaminar thalamic nuclei, mesencephalic tegmentum, nucleus basalis, and ventral tegmental area, each showed diminished connectivity in during-episode patients relative to those of postresolution patients and comparison subjects. The intralaminar thalamic nuclei and mesencephalic tegmentum have been shown to be functionally interconnected and to form part of the ascending reticular activating system, which is known to be involved in the regulation of consciousness and arousal (
14,
22). Considering that a disturbance of consciousness is an essential prerequisite for diagnosing delirium, reduced association between these two subcortical regions during an episode of delirium and its recovery after the resolution may explain some of the pathophysiology of delirium.
The striatum has been presumed to play an important role in delirium as it is one of the richest regions innervated by the acetylcholine and dopamine neurons. Antidepressant-induced (
41) or ECT-induced (
42,
43) delirium has been associated with striatal lesions. Our findings also support a pathophysiological role of the caudate in delirium in that its connectivity strengths with other subcortical regions were weakened during the episode of delirium and normalized after the resolution of delirium. The substantial and reversible aberration of functional connections among the subcortical regions supports the use or development of cholinergic/dopaminergic drugs in both the prevention and the treatment of delirium.
There are some limitations in this study. Because only patients who could cooperate with MRI scanning were recruited, selection bias should be taken into account when generalizing the findings to the patients with more severe symptoms who could not complete the scanning. Although our sample patients included all motor subtypes, connectional differences between the subtypes could not be analyzed because of the limited sample size. As logistical difficulties, including early discharge and the persistence of delirium, made it possible to obtain paired scans in only 13 patients, this reduced sample size may result in limited conclusions. In addition, the effects of antipsychotic medication could be a confounding factor, but it may be only one of diverse confounding factors of various general medical conditions in patients with delirium. A recent review reported that there was no general effect of antipsychotics on the fMRI signal, although the extent of blockade of the dopamine receptor influenced the fMRI signal (
44). It should be noted here that the number of patients treated with antipsychotics was similar between the initial and follow-up scans and that only low-dose antipsychotics were administered in both scan conditions. Finally, imaging subcortical connectivity was potentially problematic, because some regions are smaller than the spatial resolution of the fMRI. This is inevitable when studying a small structure because all fMRI analyses include a smoothing procedure, and interpreting the results from subcortical connectivity needs to be made with caution.