Marijuana has been demonized, glorified, and now increasingly “medicalized.” A well-funded effort is under way to spread the legalization of “medical marijuana” by means of referenda and state legislation beyond the 16 states and District of Columbia where it is currently legal. Dispensaries are generally set up to sell the marijuana, with restrictions varying from strict to almost nonexistent. Individuals must have a recommendation and usually a diagnosis from a physician to obtain a “medical marijuana” card.
Requiring physicians' approval of medical marijuana raises key issues for consideration by the medical profession. These include the unusual route of administration; abrogation of the role of the Food and Drug Administration (FDA); evidence of efficacy; concerns about potency, purity, and composition; effect on teenage use and marijuana dependence; marijuana side effects; concern that “medical marijuana” is a stalking horse for legalization of recreational marijuana; and most fundamentally, the role of individual physicians in the care of their patients. Brett and McCullough (
1) articulate this principle elegantly for physicians: “In managing requests for nonbeneficial services, physicians should justify their positions, invoke practice guidelines when appropriate, and offer suitable alternatives. The resulting clinical encounter reflects the physician's role as educator and enhances deliberative decision making in partnership with patients. [It] requires that physicians be committed to practicing evidence-based medicine and to lifelong learning.” The purpose of this commentary is to provide psychiatrists with an overview of the issues raised by medical marijuana and the evidence available to help them educate their patients.
No FDA-approved medication is smoked. In addition to the concerns about potential carcinogenicity (especially in heavy marijuana users [
2], but apparently not in occasional users [
3]), there is great difficulty in delivering the exact dose, if a “dose” even exists. Unlike FDA-approved medications, medical marijuana is not a specific product with controlled dosages. Medical marijuana bypasses the century-old, scientifically based drug approval procedure and the carefully regulated distribution of medications through licensed pharmacies. The FDA does not evaluate chemicals or plants like marijuana; it evaluates specific standardized products for their safety, efficacy, and purity. However, the potential therapeutic value of the cannabinoids in the marijuana plant should be harnessed to produce medications that can be approved by the FDA. The FDA approved dronabinol (Marinol), the schedule III synthetic delta-9-tetrahydrocannabinol (THC), in 1985. Sativex, a standardized marijuana-based product delivered by means of oromucosal spray and manufactured under the name Nabiximols in the United States, contains THC and cannabidiol in a 1:1 ratio and has been approved for use in the United Kingdom, Canada, and a number of other countries (
4). It is currently being evaluated in a phase 3 trial for cancer pain in the United States. Approving medications by ballot initiatives and state legislative actions sets a dangerous precedent for public health. FDA approval has usually helped keep dangerous and ineffective—but often popular—drugs off the market. Substituting a political drug approval for that protection is hazardous to the nation's health and safety.
Many medical uses for marijuana have been proposed (
5). Those indications with the most evidence include severe nausea/vomiting associated with cancer chemotherapy; cachexia associated with AIDS or cancer; spasticity secondary to neurological diseases such as multiple sclerosis (
6); pain management, especially neuropathic pain (
7); and rheumatoid arthritis. Medical marijuana legislation such as California's Proposition 215 embraces these indications “or any other illness for which marijuana provides relief.” The large majority of those with a medical marijuana card in California or Colorado, the states with the largest number of dispensaries, do not have these more serious conditions and simply claim chronic pain (
8,
9). Most of the evidence for marijuana's efficacy remains anecdotal. Further, acceptable alternatives are available for all of the above conditions, including synthetic THC agents and other non-THC FDA-approved medications. Most studies of marijuana efficacy have used oral products such as synthetic THC rather than smoked products like crude marijuana.
Prompted by the passage of California's Proposition 215, which established medical marijuana in 1996, the federal government asked the National Academy of Sciences' Institute of Medicine to study the uses of marijuana for therapeutic purposes. The Institute of Medicine concluded that there is therapeutic potential for some of the cannabinoids found in marijuana but that smoked marijuana is an unacceptable delivery system with harmful health effects. The Institute therefore recommended that clinical trials of cannabinoid drugs develop “rapid-onset, reliable, and safe delivery systems.” Moreover, the Institute stressed that clinical trials of smoked marijuana should be very limited, be short term, and be conducted under strict circumstances, such as being limited to patients who failed to benefit from approved medications (
10). The conclusions and recommendations of the Institute of Medicine are a far cry from supporting the use of smoked marijuana in medicine, as many medical marijuana advocates suggest.
Other major problems with medical marijuana are the issues of potency, purity, and composition. In the 1960s and 1970s, the average potency of seized marijuana was as low as 1% THC concentration. Now the potency of seized marijuana is up to 10% THC (
11). Today, medical marijuana dispensaries may offer marijuana with THC potency as high as 20%. The buyer has no way of knowing the accuracy of the claims or the purity of the product. Marijuana can be contaminated by molds, fungi, or herbicides. Even when potency and purity are known (e.g., the marijuana that the National Institute on Drug Abuse provides for research), how much THC the user gets is related to the depth and duration of the inhalation. An additional issue is the composition of the marijuana. The cannabis plant contains over 400 substances and over 60 cannabinoids. Of special interest is cannabidiol, a THC antagonist with antipsychotic effects (
12). Because increased cannabidiol leads to decreased available THC, some growers breed cannabidiol out.
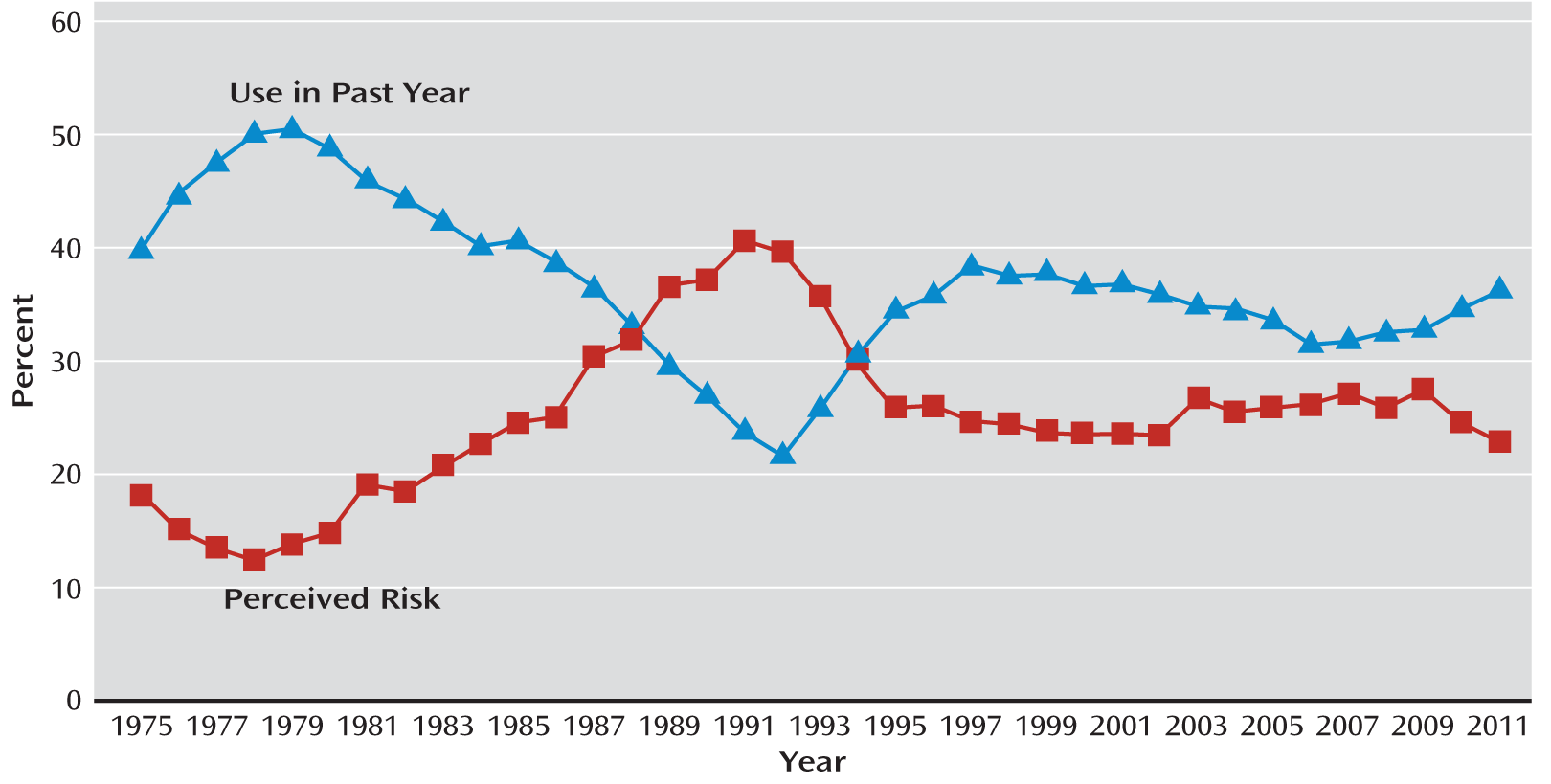
A related concern is the effect of medical marijuana, including the presence of dispensaries, on teenage marijuana use. The Monitoring the Future survey, conducted by the University of Michigan since 1975, found that the rate of marijuana use in youths is inversely related to “perceived risk” and “perceived social disapproval” (see
Figure 1). In 2011, daily 12th-grade marijuana use was at the highest level in 30 years (
13). There is also a valid concern that medical marijuana is a stalking horse for legalizing the manufacture, sale, and use of marijuana. In California, there are four referenda attempting to put marijuana legalization on the ballot for fall 2012, and seven other states are considering marijuana legalization. The proliferation of medical marijuana and legalization efforts across the country can be expected to decrease the perceptions of both risk and social disapproval of marijuana use in youths.
Medical marijuana dispensaries can be difficult to control. In some areas, marijuana dispensaries have become as ubiquitous as McDonald's or Starbucks. When cities try to regulate the number of dispensaries and their location (e.g., not within 1,000 feet of schools or playgrounds) or attempt to close dispensaries down because of surrounding criminal behavior, they may be met with a phalanx of lawyers. In some areas, schools feel surrounded by dispensaries (
14).
It would be a mistake to ignore the concerns about potential side effects when marijuana is used for either medical or recreational purposes (
15,
16). It is estimated that 9% of marijuana users will become dependent (
17), and the number of addicted individuals will likely rise as more use it. Marijuana is the most widely abused illegal drug in the United States and in the world. At present, 61% of Americans ages 12 and older who meet diagnostic criteria for substance abuse or dependence for any drug other than alcohol do so because of their marijuana use (
18,
19). Contrary to the statements of marijuana advocates, cessation of marijuana use has been shown to produce a physical withdrawal syndrome (
20–
23), with relapse after treatment as high as 71% (
24). Serious side effects related to marijuana use include, among other things, greater marijuana dependence (
25); more drugged driving with the potential for more traffic accidents (
26); short-term memory deficits; decreased concentration, attention, and information processing (
15); and aggravation of symptoms and course of schizophrenia (
27–
31), relapse of stable schizophrenia, and earlier onset of schizophrenia in vulnerable males. These side effects are more likely to occur with higher marijuana potency and earlier onset of marijuana use.
Medical marijuana laws have challenged the way physicians practice medicine by asking them to recommend to their patients the use of a schedule I illegal drug of abuse with no scientific approval, dosage control, or quality control. Several medical societies, including APA, the American Medical Association, and the American Society of Addiction Medicine, have considered the medical marijuana movement and oppose it. The American Society of Addiction Medicine specifically recommended that “its members and other physician organizations and their members reject responsibility for providing access to cannabis and cannabis-based medications until such time as they receive marketing approval from the FDA.”
How should physicians address the issue of medical marijuana in their practice? What should they tell patients seeking a recommendation? We believe that physicians should clearly explain to their patients that medical marijuana is not approved by the FDA and that it is not a standardized or purified product that has obtained scientific approval and is available in pharmacies through prescriptions. Physicians may be concerned about the malpractice or liability issues that arise from a “recommendation of use” rather than a clear prescription stating the dosage, quantity, and directions for use that accompany a regulated medication. Further, the recommendation is open-ended in that it does not state the exact conditions for which it is to be used, and the physician's responsibility for a motor vehicle accident may be unclear. Physicians should help their patients obtain approved medications for legitimate medical problems. They should also consider the greater impact of referral decisions on their communities, which have been affected by the increase in marijuana dispensaries. Many cities and counties in Colorado and California are banning dispensaries because of the chaos they create; physicians who refer patients have unwittingly but nonetheless effectively contributed to the crime and other problems created by the dispensaries. We encourage physicians as respected opinion leaders to speak out in support of the nation's science-based drug approval system and drug distribution through licensed pharmacies.