There is a long-standing debate about whether bipolar disorder and schizophrenia should be considered separate diseases or different manifestations of the same pathophysiological process. For example, genetic studies have shown shared risk genes (but not copy number variants) for bipolar disorder and schizophrenia (
1,
2). In contrast, while individuals with both disorders exhibit difficulties in community functioning, the clinical presentations are partially distinct in that patients with bipolar disorder tend to show better social connectedness when not in a mood episode relative to schizophrenia patients (
3). Cognition is a strong determinant of functioning in both disorders (
4); however, the magnitude of impairment in different domains of cognition across disorders is not well understood.
Most of our knowledge of cognitive deficits in both bipolar disorder and schizophrenia comes from studies of nonsocial cognition (e.g., attention, memory) (
5,
6). However, the degree of impairment in social cognition relative to nonsocial cognition is not well established in either disorder, and social cognition has not been systematically examined in bipolar disorder across subdomains. Social cognition refers to the ability to recognize, reason about, and appropriately respond to socioemotional information, such as the emotions, intentions, and dispositions of others (
7). Social cognition can be divided into low-level processes that involve recognition and perception of socioemotional cues, including facial expression, vocal intonation, and gestures, and high-level processes that include inferences about the mental states of others (i.e., mental state attribution), empathy, and emotional regulation (
7).
The literature on social cognition in schizophrenia has grown rapidly in the past decade. Schizophrenia patients exhibit impairments in both low- and high-level social cognitive processes (
8–
11), and their impaired social cognition is consistently related to functional outcome (
12,
13). Much less is known about social cognition in bipolar disorder, and the few studies on this topic have been largely limited to facial affect recognition and mental state attribution. Studies of facial affect perception have shown inconsistent findings in the disorder, with some studies finding intact facial affect perception and others finding impairments in bipolar patients relative to comparison subjects (
14–
16). Most studies have included relatively small patient samples with varied clinical symptoms (e.g., depressed or manic mood episode). For mental state attribution, bipolar patients in a mood episode have shown impairment (
17,
18). Although findings in remitted patients are mixed (
19,
20), a meta-analytic study found moderately impaired mental state attribution in remitted bipolar patients (
21). Thus, it remains unclear whether social cognitive impairments exist outside of mood episodes or whether these impairments would be observed across a variety of social cognitive tasks. Furthermore, neither the level nor the pattern of social cognitive performance has been directly compared across bipolar disorder and schizophrenia.
In this study, we aimed to directly compare the level and pattern of social and nonsocial cognitive performance in bipolar disorder and schizophrenia patients using behavioral tasks. First, we evaluated the performance of patients with bipolar disorder on each social cognitive task separately (emotion perception, emotion regulation, empathic accuracy, mental state attribution, and self-referential memory). Although most of the tasks have been validated in healthy adults and have been used in studies of schizophrenia patients, they have not previously been used in studies of bipolar disorder. We also explored whether subgroups of bipolar disorder patients (defined by clinical features or medication status) would perform differently on social and nonsocial cognitive tasks. Second, we examined whether bipolar disorder and schizophrenia patients would show similar patterns of performance (i.e., profiles) across social and nonsocial cognitive tasks. Third, we compared the level of impairment on social and nonsocial cognitive domains across the two disorders.
Method
Participants
Participants were 106 outpatients with bipolar disorder (N=68) or schizophrenia (N=38) and 36 healthy comparison subjects. Patients were recruited from outpatient clinics at the University of California, Los Angeles (UCLA), the Veterans Affairs Greater Los Angeles Healthcare System, and from local board and care facilities in Los Angeles. Comparison subjects were recruited through online postings.
The Structured Clinical Interview for DSM-IV (SCID) Axis I Disorders (
22) was administered to all participants to confirm their diagnostic eligibility. Patients were included if they had a DSM-IV diagnosis of bipolar I or II disorder or schizophrenia and excluded if, based on review of medical records, they had substance dependence in the past 6 months, substance abuse in the past month, or an IQ <70. Healthy comparison subjects were excluded if they had a history of schizophrenia, other psychotic disorders, bipolar disorder, recurrent major depressive disorder, substance dependence disorder, or substance abuse in the past month; a family history of psychotic disorder or bipolar disorder among first-degree relatives based on self-report; or avoidant, paranoid, schizoid, schizotypal, or borderline personality disorder, based on the Structured Clinical Interview for DSM-IV Axis II Disorders (
23). Additional exclusion criteria for all participants were a lifetime history of loss of consciousness for more than 1 hour due to head trauma; a significant neurological disorder; or insufficient fluency in English, based on a clinician’s judgment, to understand the procedures. Clinical characteristics of patients were assessed using the Hamilton Depression Rating Scale (HAM-D) (
24), the Young Mania Rating Scale (
25), and the expanded 24-item Brief Psychiatric Rating Scale (BPRS) (
26).
All patients were clinically stable, and none had experienced a mood episode in the past month. All schizophrenia patients were taking antipsychotic medications at the time of testing. In the bipolar group, at the time of testing, 41 patients were taking antipsychotic medications and 13 were taking lithium. All participants had normal or corrected-to-normal vision of at least 20/30, and none had taken a sedative or a benzodiazepine within 12 hours of testing.
All interviewers received training through the Treatment Unit of the Department of Veterans Affairs Veterans Integrated Service Network 22 Mental Illness Research, Education, and Clinical Center. SCID interviewers were trained to a minimum kappa of 0.75 for key psychotic and mood items, and symptom raters were trained to a minimum intraclass correlation of 0.80. All participants were evaluated for the capacity to give informed consent and provided written informed consent after all procedures were fully explained, according to procedures approved by the institutional review boards at UCLA and the Veterans Affairs Greater Los Angeles Healthcare System.
Social Cognitive Tasks
Facial affect recognition task.
The facial affect recognition task (
27) includes 56 color photographs of eight different individuals displaying facial expressions of six basic emotions (happy, sad, angry, afraid, surprised, and disgusted) plus neutral expressions from a standardized stimulus set (
28). On each trial, a photograph and a list of the seven possible choices for facial expression were simultaneously presented on the screen until participants made a response (participants had up to 5 seconds to respond). The dependent measure was accuracy.
Mayer-Salovey-Caruso Emotional Intelligence Test (MSCEIT), 2.0 (managing emotions component).
The managing emotions component of the MSCEIT (
29) assesses the regulation of emotions in oneself and in one’s relationships with others by presenting vignettes of various situations, along with ways to cope with the emotions depicted in these vignettes. It was administered as a part of the MATRICS (Measurement and Treatment Research to Improve Cognition in Schizophrenia) Consensus Cognitive Battery (
30). The dependent measure was a normed T score.
Empathic accuracy task.
The empathic accuracy task (
8) assesses the accuracy of empathic judgments using 12 video clips (62–137 seconds in length; six positive and six negative events), in which individuals (referred to as “targets”) discuss a positive or negative autobiographical event. While participants watched the clip, they used a 9-point scale to continuously rate how positive or negative they believed the target was feeling at each moment. The dependent measure was the correlation between participants’ ratings of the targets’ emotions and the targets’ ratings of their own emotions that were made immediately after filming, calculated in 2-second time epochs throughout each clip.
The Awareness of Social Inference Test, Part III (TASIT).
The TASIT (
10) consists of 16 videotaped scenes (15–60 seconds in length) with two or three actors appearing in each scene. The scenes involve two types of conversational exchanges, enacted as either a lie (a white lie or a sympathetic one) or sarcasm. After each scene, participants answer questions (yes/no) about the actors’ communicative intentions, such as whether the actors wanted the literal or nonliteral meaning of their messages to be believed. The dependent measures were scores for the lie and sarcasm subscales.
Self-referential memory task.
The self-referential memory task (
9) assesses self-referential memory using two task phases: encoding and recognition. During encoding, a trait adjective and a cue on trial type were presented for 2.3 seconds, and for 78 positive and 78 negative trait adjectives, participants made a judgment (yes/no) as to whether a trait word was uppercase (physical condition), socially desirable (general social condition), or described themselves (self-condition). Ten minutes later, a recognition memory test was administered in which participants indicated whether or not they recognized 156 seen words and 156 unseen words. The dependent measure was a measure of sensitivity (A′) for each encoding condition.
Nonsocial Cognitive Tasks
MATRICS Consensus Cognitive Battery (MCCB).
The MCCB (
30) assesses seven separate cognitive subdomains, including one measure of social cognition, the managing emotions component of the MSCEIT. We included the six nonsocial cognitive tasks: speed of processing, attention/vigilance, working memory, verbal memory, visual memory, and reasoning/problem solving. The dependent measures were normed T scores for each cognitive subdomain.
Statistical Analysis
For each social and nonsocial cognitive task, we excluded outliers that were three standard deviations below the mean within each group (one bipolar patient for the facial affect recognition task; one bipolar patient for the empathic accuracy task; two bipolar patients and one schizophrenia patient for the lie subscale of the TASIT; one comparison participant for the sarcasm subscale of the TASIT; and one bipolar patient, one schizophrenia patient, and one comparison participant for the self-referential memory task).
First, we evaluated social cognitive performance in bipolar patients for each task using a series of univariate analyses of variance (ANOVAs) for tasks with one dependent variable and repeated-measures ANOVAs for tasks with more than one dependent variable. Significant main effects or interactions were followed up with paired contrasts corrected for multiple comparisons using Bonferroni corrections. We conducted additional analyses to investigate the extent to which subgroups of bipolar patients performed differently on each social and nonsocial cognitive task using univariate ANOVAs and repeated-measures ANOVAs for the following comparisons: bipolar I patients (N=46) compared with bipolar II patients (N=22), bipolar I patients with a history of psychosis (N=15) compared with bipolar I patients without a history of psychosis (N=31), and bipolar patients taking antipsychotic medication (N=41) compared with those not taking antipsychotic medication (N=27).
Second, we evaluated patterns of performance across tasks (i.e., profiles) within the domains of social and nonsocial cognition in bipolar disorder and schizophrenia patients. To do so, we converted each social and nonsocial cognitive dependent variable into a z score using the mean and standard deviations for comparison participants. For the TASIT, we included both the lie and sarcasm subscales because they load on separate factors (
31). For the self-referential memory task, we included the self condition, which reflects self-referential processing (
9). We then compared the profiles across the three study groups by examining group-by-task interactions in two separate repeated-measures ANOVAs, one for the social and one for the nonsocial cognitive performance variables.
Third, we examined the overall level of impairment in social and nonsocial cognitive domains in bipolar patients compared with schizophrenia patients. To do so, we generated composite social and nonsocial cognitive scores (the average z score across all subdomains) and compared the groups using a repeated-measures ANOVA. All results at a p level <0.05 were considered significant, unless otherwise noted.
Results
Demographic and Clinical Characteristics of Participants
The three study groups were comparable on age, gender, and parental education but not on personal education (
Table 1). Schizophrenia patients showed lower levels of education compared with bipolar patients and comparison participants. For clinical symptoms, both patient groups were comparable on HAM-D scores, but schizophrenia patients showed higher scores on the BPRS and the Young Mania Rating Scale compared with bipolar patients. Higher Young Mania Rating Scale scores for schizophrenia patients resulted from higher ratings on one item assessing the content of thought (grandiose or paranoid ideas, delusions, and hallucinations).
Performance of Bipolar Patients on Social Cognitive Tasks
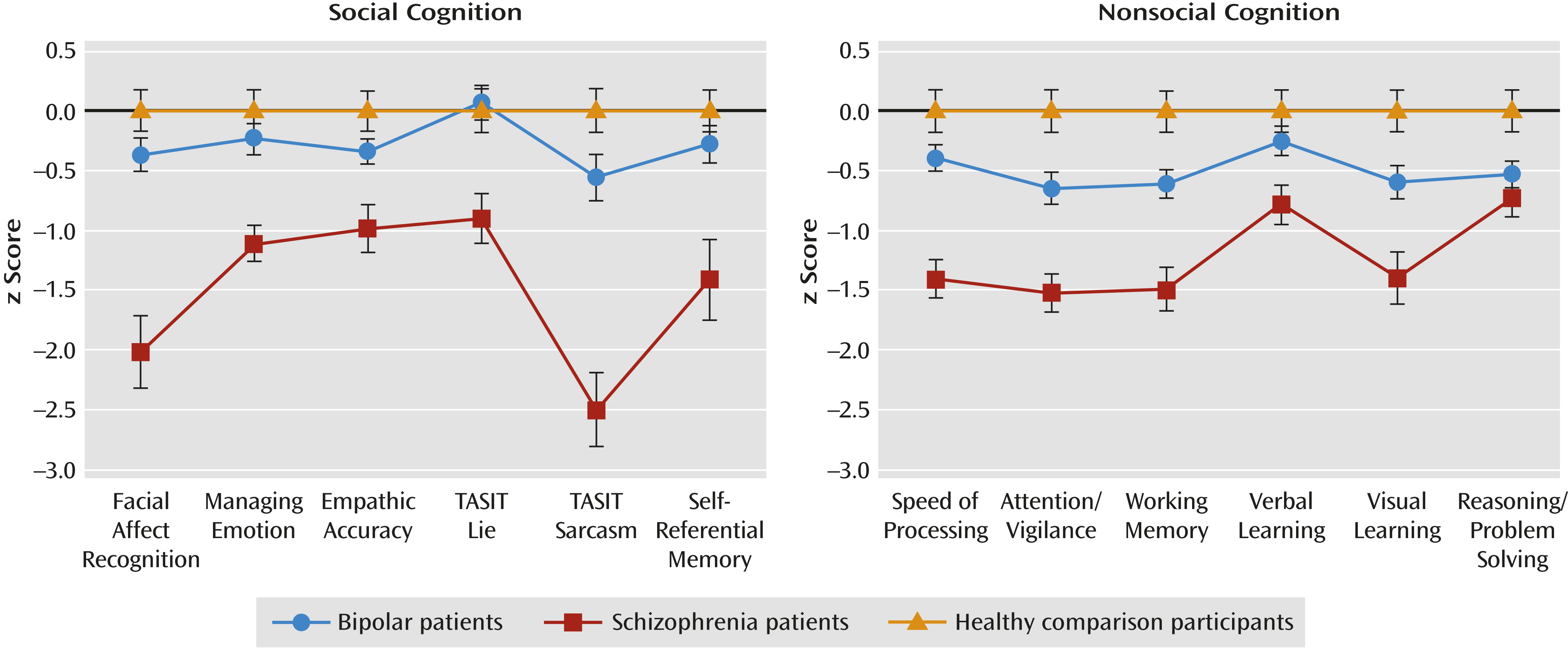
Performance on social cognitive tasks in the three study groups is summarized in
Table 2, and effect sizes are listed for the differences between groups. Notably, bipolar patients did not differ significantly from comparison participants on any task. Three tasks (facial affect recognition, managing emotions [from the MSCEIT], and empathic accuracy) showed a main effect of group in which schizophrenia patients performed significantly worse than bipolar patients and comparison participants, who did not differ from each other. Results from the TASIT, with subscale as a within-subject factor and group as a between-subject factor, revealed a significant main effect of group (F=38.71, df=2, 124, p<0.001) and a significant group-by-subscale interaction (F=3.97, df=2, 124, p<0.05). On both the lie and sarcasm subscales, schizophrenia patients performed worse than bipolar patients and comparison participants, who did not differ from each other. While bipolar patients performed comparably on both subscales, schizophrenia patients showed worse performance on the sarcasm subscale than on the lie subscale, and comparison participants showed a reverse pattern. The self-referential memory task, with the encoding condition as a within-subject factor and group as a between-subject factor, revealed a significant main effect of condition (F=213.38, df=2, 262, p<0.001) and a significant condition-by-group interaction (F=8.54, df=4, 262, p<0.001). All groups performed best in the self condition and poorest in the physical condition. In the self and general social conditions, schizophrenia patients performed worse than bipolar patients and comparison participants, who did not differ from each other. The groups did not differ in performance in the physical condition.
In the nonsocial cognitive tasks, group effects were significant for each task (
Table 2). For these tasks, the difference between bipolar patients and comparison participants was significant for three subdomains (attention/vigilance, working memory, and reasoning and problem solving) and fell short of significance for visual learning (p=0.06).
The majority of bipolar patients (N=52) were euthymic at the time of testing (defined by a HAM-D score <15 and a Young Mania Rating Scale score <12) (
32). When we limited the analyses to these patients and compared their performance on social and nonsocial cognitive tasks with that of schizophrenia patients and comparison participants, results were unchanged. Within each patient group, none of the social and nonsocial cognitive performances were significantly correlated with clinical symptoms (i.e., total scores for BPRS, HAM-D, and Young Mania Rating Scale). Finally, we did not observe any consistent differences among subgroups of patients with bipolar disorder (bipolar I patients compared with bipolar II patients, bipolar I patients with a history of psychosis compared with bipolar I patients without a history of psychosis, and bipolar patients taking antipsychotic medication compared with those not taking antipsychotic medication) (
Table 3; see also Table S1 in the data supplement that accompanies the online edition of this article). Additional analyses of group comparisons of social cognitive performance among patients with bipolar I disorder with a history of psychosis, schizophrenia patients, and comparison participants are provided in the online data supplement.
Patterns of Social and Nonsocial Cognitive Performance Across Groups
The profile graphs for social and nonsocial cognitive tasks are presented in
Figure 1. On social cognitive tasks, bipolar patients and comparison participants showed a similar performance profile. The profile for schizophrenia patients differed significantly from those of bipolar patients and comparison participants, mainly because of the relatively large impairment of schizophrenia patients on the facial affect recognition task and the sarcasm subscale of the TASIT (see the online data supplement). For nonsocial cognition, bipolar patients and comparison participants also showed similar performance profiles. Schizophrenia patients had a different profile from both bipolar patients and comparison participants that appeared to be due to relatively smaller group differences on verbal learning and reasoning/problem solving (see the online data supplement).
Relative Impairment Level for Social and Nonsocial Cognitive Domains
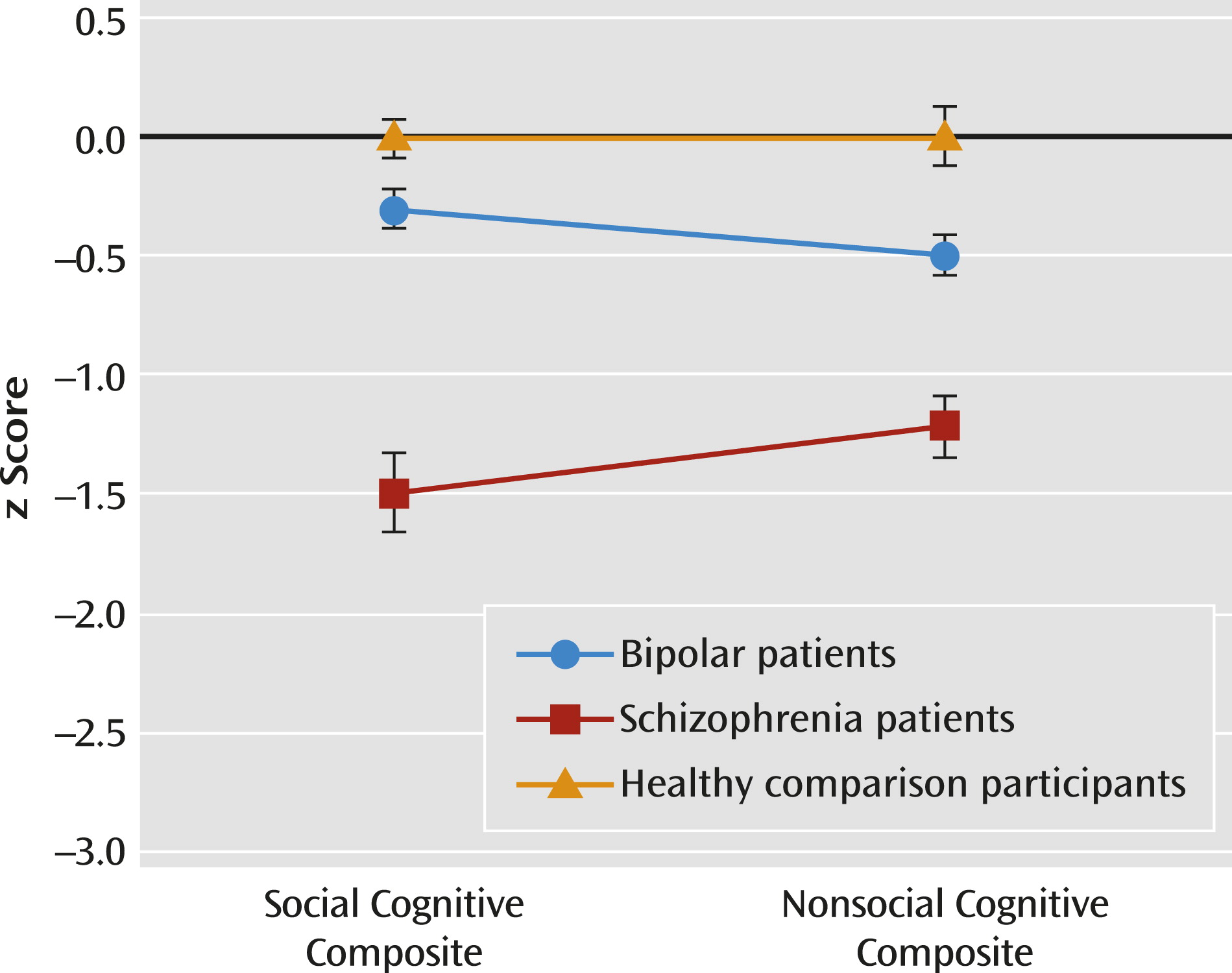
Using composite scores for each cognitive domain, a 2×3 (social cognitive/nonsocial cognitive and group) repeated-measures ANOVA revealed a significant main effect of group (F=43.54, df=2, 132, p<0.001) and interaction (F=5.53, df=2, 132, p<0.01) (
Figure 2). Across cognitive domains, bipolar patients showed intermediate performance that was significantly different from that of schizophrenia patients and comparison participants. For social cognition, schizophrenia patients performed worse than both bipolar patients and comparison participants (all p values <0.001 for both groups), and the bipolar group did not differ from the comparison group. For nonsocial cognition, schizophrenia patients performed worst and comparison participants performed best; bipolar patients showed intermediate performance significantly different from that of comparison subjects (p<0.01) and schizophrenia patients (p<0.001). Impaired social cognition in schizophrenia patients was not explained by their nonsocial cognitive impairments (see the online data supplement). To examine the relative level of impairment between bipolar and schizophrenia patients, we conducted a follow-up repeated-measures ANOVA and found a significant group effect (F=47.60, df=1, 101, p<0.001) and group-by-domain interaction (F=10.46, df=1, 101, p<0.005). Bipolar patients showed significantly better overall performance on social cognition than nonsocial cognition (p<0.05), whereas schizophrenia patients showed a significant reverse pattern (p<0.05). Finally, an exploratory discriminant analysis showed that social and nonsocial cognitive performance discriminate among the three groups, as well as the two patients groups, better than each cognitive domain alone (see the online data supplement).
Discussion
This study evaluated the magnitude and pattern of social and nonsocial cognitive performance of bipolar patients compared with that of schizophrenia patients and healthy comparison participants. In contrast to previous studies of bipolar disorder, we examined the performance of bipolar patients, none of whom were in a mood episode, on multiple domains of social cognition. Bipolar patients performed comparably to comparison participants on each social cognitive task, whereas schizophrenia patients showed impaired social cognitive performance compared with both bipolar patients and the comparison group. For both the social and nonsocial cognitive profiles, bipolar patients did not differ from comparison participants, but schizophrenia patients differed in pattern from both the bipolar and comparison groups. Finally, in a comparison of the relative impairments across social and nonsocial cognitive domains using a composite score, bipolar patients showed intermediate performance between schizophrenia patients and comparison subjects across domains. Additionally, bipolar patients performed significantly better on social relative to nonsocial cognitive domains, whereas schizophrenia patients showed the opposite pattern. Impairment in schizophrenia patients on nonsocial cognition appeared to be similar to the level of impairment (one to two standard deviations) seen commonly in clinical trials (
33).
Our finding of relatively less impaired social cognition compared with nonsocial cognition in bipolar disorder is consistent with findings from a recent study that used the MCCB to assess cognitive performance in patients with bipolar I disorder and did not find a significant group difference on the social cognitive task (
34). Our finding of less impaired social cognition in bipolar patients raises a question about the key determinants of functional outcome in bipolar disorder. Nonsocial cognition is a determinant of functioning in both bipolar disorder and schizophrenia (
4,
35,
36). In schizophrenia, social cognition mediates the relationship between nonsocial cognition and functioning and also explains the independent variance of functioning (
12,
13). Our findings suggest that social cognition may be less of a determinant of functioning in bipolar disorder than it is in schizophrenia. If so, nonsocial cognitive remediation rather than social cognitive interventions may be better suited for bipolar disorder, whereas both types of interventions may be beneficial for schizophrenia.
Some aspects of nonsocial cognition are considered to be endophenotypes for schizophrenia and bipolar disorder (
5,
37). Social cognitive impairment is also a promising endophenotype for schizophrenia because it is found in first-episode patients and in individuals at risk for schizophrenia (
38) and is stable over time (
12). The relatively intact social cognition in bipolar patients in our study suggests that it might not serve as a promising endophenotype for this disorder. Similarly, social cognition profiles in bipolar disorder may be state related, while nonsocial cognition profiles could be trait related. Although we did not find associations between clinical symptoms and social cognitive performance, bipolar patients had relatively low symptom levels, and the majority were euthymic. Studies with longitudinal designs could test whether social cognitive performance in bipolar disorder fluctuates as clinical symptoms change.
Our study has some limitations. All of the patients with bipolar disorder and schizophrenia were taking psychotropic medications; however, we did not observe differential performance in subgroups of bipolar patients based on the presence or absence of antipsychotic use. Similarly, we did not find any differential performance in subgroups of bipolar patients based on clinical features, although the sample sizes of the subgroups were relatively small. The patient groups tended to be older with relatively chronic illness; hence it is unclear whether the same findings would be observed in recent-onset patients. Finally, while social cognitive tasks assessed multiple areas, all of them were behavioral tasks, and emotional regulation was not well covered compared with other areas.
Although social and nonsocial cognition are considered separate but overlapping constructs (
39), our finding of relatively intact social cognition and impaired nonsocial cognition in remitted bipolar patients suggests that the two domains are not redundant and that social cognitive impairment does not necessarily follow deficits in nonsocial cognition. Our finding of relatively intact social cognition in the presence of impaired nonsocial cognition in bipolar disorder, opposite to the pattern seen in schizophrenia, raises questions about how this pattern emerged. One possibility is that bipolar patients have compensatory mechanisms that protect social cognitive circuits in the context of impaired nonsocial cognition and that these compensatory mechanisms are missing in schizophrenia. Alternatively, the extent of compromised neural circuits may be greater in schizophrenia than in bipolar disorder, and key regions involved with social cognition (e.g., the medial prefrontal cortex and temporo-parietal junction) may be less affected in bipolar disorder than those involved in nonsocial cognition. Applying neuroimaging methods from social neuroscience to studies of bipolar disorder and schizophrenia may help to elucidate such possibilities.
Acknowledgments
The authors thank Crystal Gibson, Christen Waldon, Amanda Bender, and Cory Tripp for assistance with data collection and Gerhard S. Hellemann, Ph.D., for statistical advice.