“A rose is a rose is a rose” (
1). For psychiatric diagnosis, we still interpret this line as Robins and Guze did for their Research Diagnostic Criteria—that reliability is the first test of validity for diagnosis (
2). To develop an evidence-based psychiatry, the Robins and Guze strategy (i.e., empirically validated criteria for the recognizable signs and symptoms of illness) was adopted by DSM-III and DSM-IV. The initial reliability results from the DSM-5 Field Trials are now reported in three articles in this issue (
3–
5). As for all previous DSM editions, the methods used to assess reliability reflect current standards for psychiatric investigation (
3). Independent interviews by two different clinicians trained in the diagnoses, each prompted by a computerized checklist, assessment of agreement across different academic centers, and a pre-established statistical plan are now employed for the first time in the DSM Field Trials. As for most new endeavors, the end results are mixed, with both positive and disappointing findings.
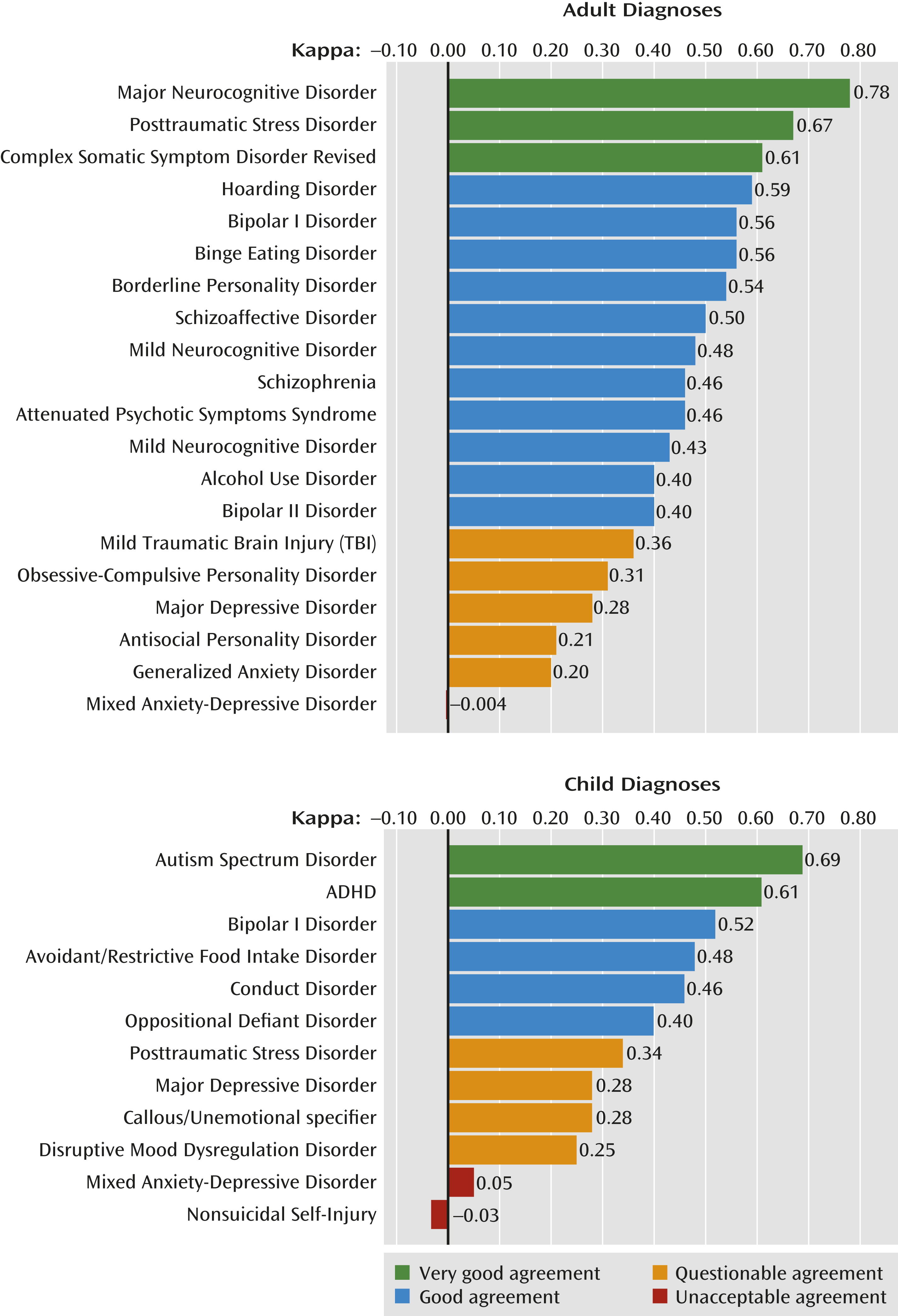
The kappa statistic that is used for the analysis may not be familiar to most clinicians. For illustration, if an illness appears in 10% of a clinic’s patients and two colleagues agree on its diagnosis 85% of the time, the kappa statistic is 0.46, similar to the weighted composite statistic for schizophrenia in this DSM-5 Field Trial (
Figure 1). Schizophrenia was radically changed in DSM-III and modified again in DSM-IV because of discrepancies worldwide in its diagnosis. Now, the problem in distinguishing schizophrenia, bipolar disorder, and schizoaffective disorder—the crux of the discrepancies—has largely resolved, and all three conditions have good kappa statistics.
The questionable reliability of major depressive disorder, unchanged from DSM-IV, is obviously a problem. Major depressive disorder has always been problematic because its criteria encompass a wide range of illness, from gravely disabled melancholic patients to many individuals in the general population who do not seek treatment. Although symptom severity on the Hamilton Depression Rating Scale distinguishes those patients who respond more specifically to pharmacotherapy, the DSM-IV criteria do not capture that distinction (
6). A second problem not resolved by the DSM-IV criteria is the common co-occurrence of anxiety, which markedly diminishes the effects of antidepressant treatment (
7). The DSM-5 work group decided not to change the criteria for major depressive disorder from DSM-IV and instead created other diagnoses for the mixture between anxiety and depression. However, these efforts did not improve the poor reliability of DSM-IV depression; “mixed anxiety and depression” has a kappa of 0. Clinicians often use patients’ self-rating on the Beck Depression Inventory as an indicator of severity. The dimensional cross-cutting domains in this field trial similarly rely on self-rating (
5). For depression there are two domains and the intraclass correlations, which are similar to the kappa statistic, for adult patients rating and rerating themselves and for parents rating their children; all exceed 0.6. Future revisions will likely need to integrate the many factors—patient self-ratings, cognitive biases, co-occurring anxiety, and vegetative symptoms—that guide treatment selection, prognosis, and assessment of suicide risk.
Experienced clinicians have severe reservations about the proposed research diagnostic scheme for personality disorder, and its applicability to clinical practice has yet to be determined (
8). Most of the personality disorder diagnoses did not do well in the field trial. Antisocial and obsessive-compulsive personality disorders had questionable or inconclusive reliability, and other types like narcissistic and schizotypal personality disorder were seen too infrequently to be assessed. The success of borderline personality disorder is nonetheless a major step forward. DSM-III relegated most personality disorders to axis II, radically severing one of psychiatry’s most venerable roots. But clinicians recognized that character pathology, despite its seeming stability, was both quite disabling and amenable to treatment. Borderline personality disorder now emerges as a major diagnosis in its own right with good diagnostic reliability.
Unstable mood, a cardinal feature of borderline personality disorder in adulthood, is also the prominent feature in childhood of a new disorder, disruptive mood dysregulation disorder. This disorder has a more modest kappa statistic. Disruptive mood dysregulation disorder was more reliably assessed in the inpatient setting where it was examined, as was borderline personality disorder early in its history. Perhaps as clinical experience with this new childhood diagnosis increases, its diagnostic performance will improve. Reliability of ADHD and childhood bipolar disorder diagnoses, which had been problematic particularly when irritability was present, likely benefitted from the alternative of disruptive mood dysregulation disorder; both have good kappa statistics. The newly reorganized autism spectrum disorder, also subject of much previous debate, has a very good kappa, although the trials did not include children under 6 years old.
PTSD is another historic accomplishment, with a kappa of 0.67. The DSM series was initiated because “the ‘psychoneurotic label’ had to be applied to men reacting briefly with neurotic symptoms to considerable stress; individuals who…were not ordinarily psychoneurotic” (
9). Four editions and 60 years later, PTSD is now a reliable diagnosis for a disorder that might have been dismissed as pathologizing normal behavior. Other new or redefined diagnoses have been introduced with good reliability: major neurocognitive disorder, hoarding disorder, complex somatic symptoms disorder, and binge eating disorder, in addition to those already discussed.
The field trials required that a diagnosis be reached from a single patient interview with minimal collateral information. For a general psychiatric practice, the diagnostic reliability data suggest that two-thirds of patients will receive a reliable DSM-5 principal diagnosis at the first visit. These common, reliable diagnoses are childhood ADHD, PTSD, borderline personality disorder, and alcohol use disorder. The one-third of patients with mild TBI or major depressive disorder may not have a reliable diagnosis from a single interview. Of course, this estimate—derived by combining Table 1 (sample weights in an adult outpatient setting, inserting childhood ADHD as the “other diagnosis” category) with Tables 2 and 4 (reliability of adult and childhood diagnoses [4])—will be different for each clinical setting. Robins and Guze introduced an “undiagnosed” category to urge that patients be re-examined over time when their initial symptoms do not lead to an unambiguous diagnosis. The DSM-5 Field Trials did not examine the increased reliability derived from the same treating clinician assessing the patient over time as the illness unfolds.
“A rose is a rose is a rose is a rose” had deeper meaning for Gertrude Stein, to do not only with the classification of the flower but also with its enduring essence (
10). Understanding the natural course of a disorder, its response to treatment, and its impact on the life of the individual are the reasons that we strive to make reliable diagnoses, but a single diagnostic interview, regardless of how reliable, does not capture the essence of what is happening to a patient. If there are lessons for clinicians and patients and families reading these field trials, perhaps the most important one is that accurate diagnosis must be part of the ongoing clinical dialogue with the patient.
The improvement of diagnosis is also ongoing. Future tests need to consider clinical utility in actual treatment situations and the reliability and practicality of applying the new criteria outside academic medical centers. Solo practitioners and mental health clinics may not have resources for the level of training that the field trials required. The patients were required to speak and read English, although some were bilingual. Reliability may not be the same for patients who have lower levels of education or for whom English is not their most fluent language. The findings of these field trials will be used to make further improvements, and hence the final criteria may change and require further testing after DSM-5 publication. Like its predecessors, DSM-5 does not accomplish all that it intended, but it marks continued progress for many patients for whom the benefits of diagnoses and treatment were previously unrealized.