In 2004, the Food and Drug Administration issued a black box warning regarding the use of antidepressants in the pediatric population (
1). However, overwhelming evidence supports the efficacy of selective serotonin reuptake inhibitors (SSRIs) in treating mood and anxiety disorders in this population (
2). Nevertheless, studies on the use of SSRIs suggest transient, age-delimited effects on suicidal ideation (
3,
4) as well as long-lasting effects on the developing brain in rodents (
5,
6). These issues are particularly salient in humans as a result of the protracted development of the human brain (
7,
8). Furthermore, in rodents, specific alterations in the serotonin transporter (SERT) or serotonin 1A (5-HT
1A) receptor have been shown to affect neuronal development (
9), and short-term SSRI exposure during a time-sensitive developmental window appears to induce long-lasting effects on serotonergic functioning and anxious behavior into adulthood (
5,
10,
11). It should be noted that both emotional experiences and environmental conditions may also affect brain development and that such changes could occur independently or synergistically with any effect of SSRIs on development (
12). Therefore, it is notable that no prospective study has examined the long-term effects of SSRIs on the developing primate brain.
Monkeys arguably provide the best model of human brain function related to psychiatric disorders because of similarities in brain structure, social organization, and protracted development (
13). Maternal separation of infant monkeys is a well-established animal model of early-life stress, which in humans predisposes to the development of mood and anxiety disorders later in life (
14,
15). Similarly, monkeys with a history of maternal separation display both behavioral and serotonergic abnormalities similar to those seen in anxious and depressed humans (
16–
18).
Although SSRIs rapidly increase 5-HT concentrations in the synapse by inhibiting SERT, their therapeutic effects are typically delayed by about 8 weeks. Animal studies suggest that one potential explanation for this delay is the time required for 5-HT
1A autoreceptors in the raphe to desensitize, thereby leading to increased release of 5-HT in terminal areas (
19). Thus, SERT and the 5-HT
1A receptor are two important serotonergic markers, both direct and indirect, in assessing the effects of long-term SSRI treatment.
The present study sought to answer one primary and two secondary questions regarding the effects of SSRIs on the developing primate brain. First, does long-term, prepubertal fluoxetine treatment have effects on the serotonergic system that are discernible in adulthood? Second, do these effects vary based on rearing conditions, i.e., whether the monkeys were maternally separated or normally reared? Third, does fluoxetine induce any long-lasting behavioral changes into adulthood? To answer these three questions, 32 differentially reared monkeys received either fluoxetine or placebo from age 2 to 3 years, which corresponds to the prepubertal period. Monkeys were scanned at ∼4.7 years with two serotonergic positron emission tomographic (PET) radioligands: [11C]DASB for SERT and [11C](R)-RWAY for 5-HT1A receptors.
Method
Study Design
At birth, 32 male rhesus monkeys (
Macaca mulatta) from four annual birth cohorts were randomly assigned to maternally separated or normally reared conditions (i.e., eight newborns from each of 4 years were equally divided between the two rearing conditions). Maternal separation consisted of the standard protocol as previously described (
20) (see supplemental methods in the
data supplement that accompanies the online edition of this article).
At 2 years of age, half of each rearing group was randomly assigned to receive either fluoxetine or placebo. Although the allometric scaling of doses between species is controversial, most pharmacologists agree that the best index of whether one has corrected for differences in absorption and disposition of a medication is the similarity of the resulting plasma concentrations across species. Thus, we selected doses of fluoxetine for this study that produced concentrations of fluoxetine and norfluoxetine (a pharmacokinetic measure) and 5-hydroxyindoleacetic acid (5-HIAA; a pharmacodynamic measure) that were similar to those concentrations seen in human patients (
21,
22). Fluoxetine (3 mg/kg/day) or placebo was administered orally in mashed bananas for 1 year. This dosage results in steady state fluoxetine concentrations of ∼70 ng/mL and norfluoxetine concentrations of ∼220 ng/mL, both of which are in the clinical range for children (
23) (see methods and Figure S1 in the online
data supplement). A number of pharmacological agents were used as part of routine veterinary care for the monkeys. For example, all monkeys received intramuscular injections of ketamine (7 mg/kg) and xylazine (6 mg/kg) four times for MRI scans. In addition, monkeys received telazol (2–6 mg/kg) a total of 8–10 times over the course of the study; this agent was administered prior to blood draws and CSF taps. All monkeys received twice daily feedings and access to water ad libitum. The study was approved by the Animal Care and Use Committee of the National Institute of Mental Health.
Positron Emission Tomography (PET) Scans
All monkeys (mean age=4.7 years, SD=0.6; mean weight=7.4 kg, SD=1.5) underwent 120-minute dynamic scans on a Focus 220 (Siemens Medical Solutions, Knoxville, Tenn.) using two PET radioligands: [
11C]DASB to label SERT and [
11C](
R)-RWAY to label 5-HT
1A receptors (
24,
25). The injected activity, specific activity, and injected mass dose of both radioligands were similar for monkeys that were administered either placebo or fluoxetine (see Table S1 in the online
data supplement).
Calculation of Binding Potential (BPND) Using a Reference Tissue Model
Binding potential
(BPND), which is the ratio at equilibrium of specific binding to nondisplaceable uptake in the brain, was used to measure receptor density (
26). Binding potential was calculated with a reference tissue model, using cerebellar white matter as the reference region for both radioligands. With regard to the location of the SERT and 5-HT
1A receptors, we a priori selected only three regions: the raphe, the hippocampus, and the neocortex. The raphe contains serotonergic neurons with high densities of SERT and somatodendritic 5-HT
1A autoreceptors. Both the hippocampus and the neocortex primarily have 5-HT
1A receptors postsynaptically.
Binding potential was calculated in three steps 1): coregistering PET and MR images in template space, 2) generating time-activity curves, and 3) performing kinetic analysis. Dynamic PET images were coregistered to averaged MRI templates from six monkeys in standardized space. Time-activity curves were generated using predefined regions of interest for both the neocortex and the hippocampus (
27) and manually for the raphe (
28). For the neocortex, five different cortical regions were combined and weighted for volume: the frontal, cingulate, temporal, parietal, and occipital cortices. The raphe (volume=110 mm
3) was drawn using a circular region of interest directly on three slices (one midsagittal and two adjacent) of the summed PET images. Radioactivity concentrations were expressed as standardized uptake value, which normalizes for weight and injected activity. Kinetic analyses were performed using the multilinear reference tissue model with two parameters, which requires a priori estimation of
k2 of the cerebellum (
k2′), the clearance rate constant from cerebellum relative to a region of specific binding. Using the multilinear reference tissue model,
k2′ values were obtained from the cerebellum relative to the thalamus for [
11C]DASB and from the cerebellum relative to the neocortex for [
11C](
R)-RWAY. [
11C]DASB binding had a good fit with a one-tissue compartment model. [
11C](
R)-RWAY binding had a good fit with a two-tissue compartment model (
27,
29). As such, the start times (
t*) were set to 0.25 minutes for [
11C]DASB and 50 minutes for [
11C](
R)-RWAY. Parametric images were generated using PMOD 3.0 (PMOD Technologies, Zurich, Switzerland).
Voxel-Wise Analysis
An exploratory voxel-wise analysis of the whole brain was performed using parametric images and statistical parametric mapping (SPM8, Wellcome Trust Centre for Neuroimaging, U.K.). Parametric images were generated using the multilinear reference tissue model with two parameters, smoothed to full width at half maximum of 4 mm and analyzed using a factorial design in SPM. Statistical parametric maps were initially thresholded at uncorrected p<0.05, and an exploratory stringent Gaussian random field theory cluster level (i.e., family-wise error) correction for multiple comparisons was applied.
Social Behavior
Peer social behavior was evaluated in a series of round robin tests that took place between 4 and 8 months after the initiation of drug administration and then again 2–6 months after drug cessation (see supplemental methods in the online
data supplement for further details of the ethogram). Behaviors from the ethogram were consolidated into nine composite measurements: locomotion, stereotypy, passivity, affiliation (physical proximity or grooming), dominance, submissiveness, coo vocalizations, bark vocalizations, and social (attack or bite) or nonsocial (cage shake) aggression. However, only eight of these were analyzed as a result of insufficient variability for one of the nine measures (aggression). Intra- and interrater reliability were greater than 0.85 on all scored behaviors.
Statistics
We used rearing-by-drug analyses of variance (ANOVA) to examine both interaction and main effects in the PET studies and a rearing-by-treatment-by-period ANOVA for each of the eight behavioral composite measurements for the behavioral studies. For both PET and behavioral studies, we report the uncorrected p value, the correction factor, and the corrected p value. We used IBM SPSS 19.0.0.1 (
http://www-01.ibm.com/software/analytics/spss/) for statistical analysis.
Results
Selecting Dosage of Fluoxetine
To select a dosage of fluoxetine to mimic that used therapeutically in humans, we conducted a pilot study in a separate group of 12 monkeys that received three doses of fluoxetine (0.5, 2, or 3 mg/kg) in an attempt to approximate the levels of drug exposure in humans (see Figure S1 in the online
data supplement) (
14,
15). Specifically, we measured the plasma concentrations of drug and the expected decrease of 5-HIAA concentrations in CSF (
22). These 12 monkeys received oral fluoxetine for 3–4 weeks. Blood and CSF were sampled 24 hours after the last drug administration. The lowest dosage (0.5 mg/kg) resulted in undetectable plasma concentrations of fluoxetine and norfluoxetine and no changes in 5-HIAA concentrations in CSF. Compared with levels in humans treated chronically with 20 mg/day, the intermediate dosage (2 mg/kg) generated lower plasma concentrations of fluoxetine and norfluoxetine. Again, compared with levels in humans treated chronically with 20 mg/day, the 3 mg/kg dosage generated lower plasma concentrations of fluoxetine but comparable plasma concentrations of norfluoxetine (see Figure S1 in the online
data supplement). At both 2 and 3 mg/kg of fluoxetine, 5-HIAA concentrations in CSF were reduced in monkeys relative to placebo, although not to the extent reported in humans (see Figure S2 in the online
data supplement). Based on these pilot data, as well as previously published studies, we selected the 3 mg/kg dosage because it provided similar pharmacokinetic measures as humans taking 20 mg/day, albeit slightly lower pharmacodynamic measures.
To confirm that these pharmacokinetic measures were achieved during the study, we measured plasma concentrations of drug and its active metabolite in the first cohort of eight monkeys. The concentrations were measured during and after the treatment period. During drug administration, the concentrations were 41 ng/mL (SD=25.2) for fluoxetine and 159 ng/mL (SD=78.4) for norfluoxetine. In the placebo group, concentrations were undetectable. Eight weeks after drug administration, concentrations of both fluoxetine and norfluoxetine were also undetectable in either the drug- or placebo-administered groups.
PET Imaging
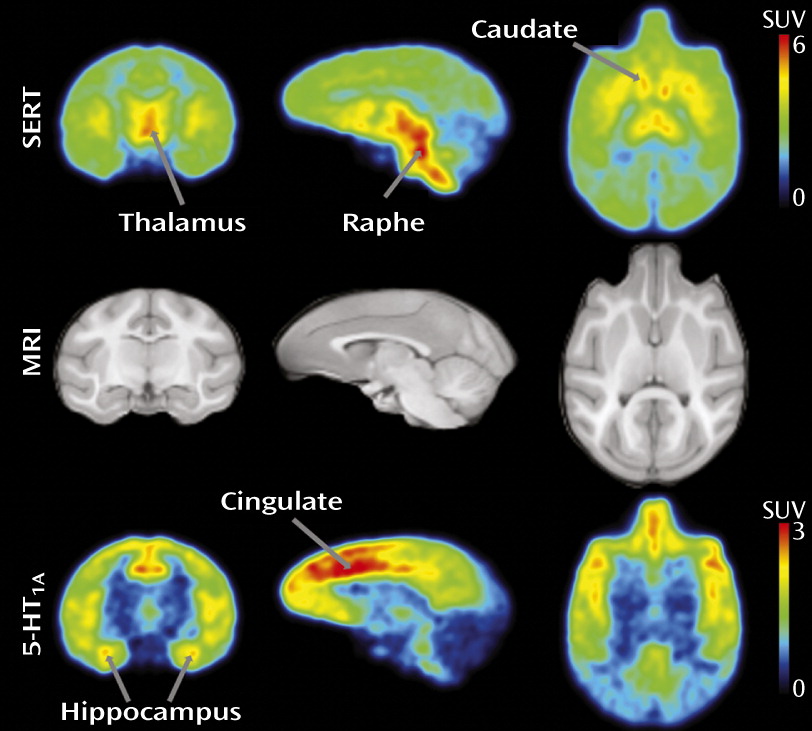
For both [
11C]DASB and [
11C](
R)-RWAY, the distribution and time course of brain uptake were similar to those observed in previous studies (
18,
27). [
11C]DASB uptake in the monkey brain reflected the known distribution of SERT, with high binding in the raphe, thalamus, and caudate (
Figure 1, top row). [
11C](
R)-RWAY uptake in the monkey brain reflected the known distribution of 5-HT
1A receptors, with high binding in the cingulate cortex and hippocampus (
Figure 1, bottom row). With regard to time course of uptake for both radioligands, higher density regions had later times of peak uptake, reflecting the greater amount of radioligand that had to be delivered to achieve equilibrium binding. For both radioligands, uptake in cerebellar white matter (the reference region) peaked early and was similar between groups of animals, the latter fulfilling a requirement to use reference tissue modeling.
Serotonin Transporter
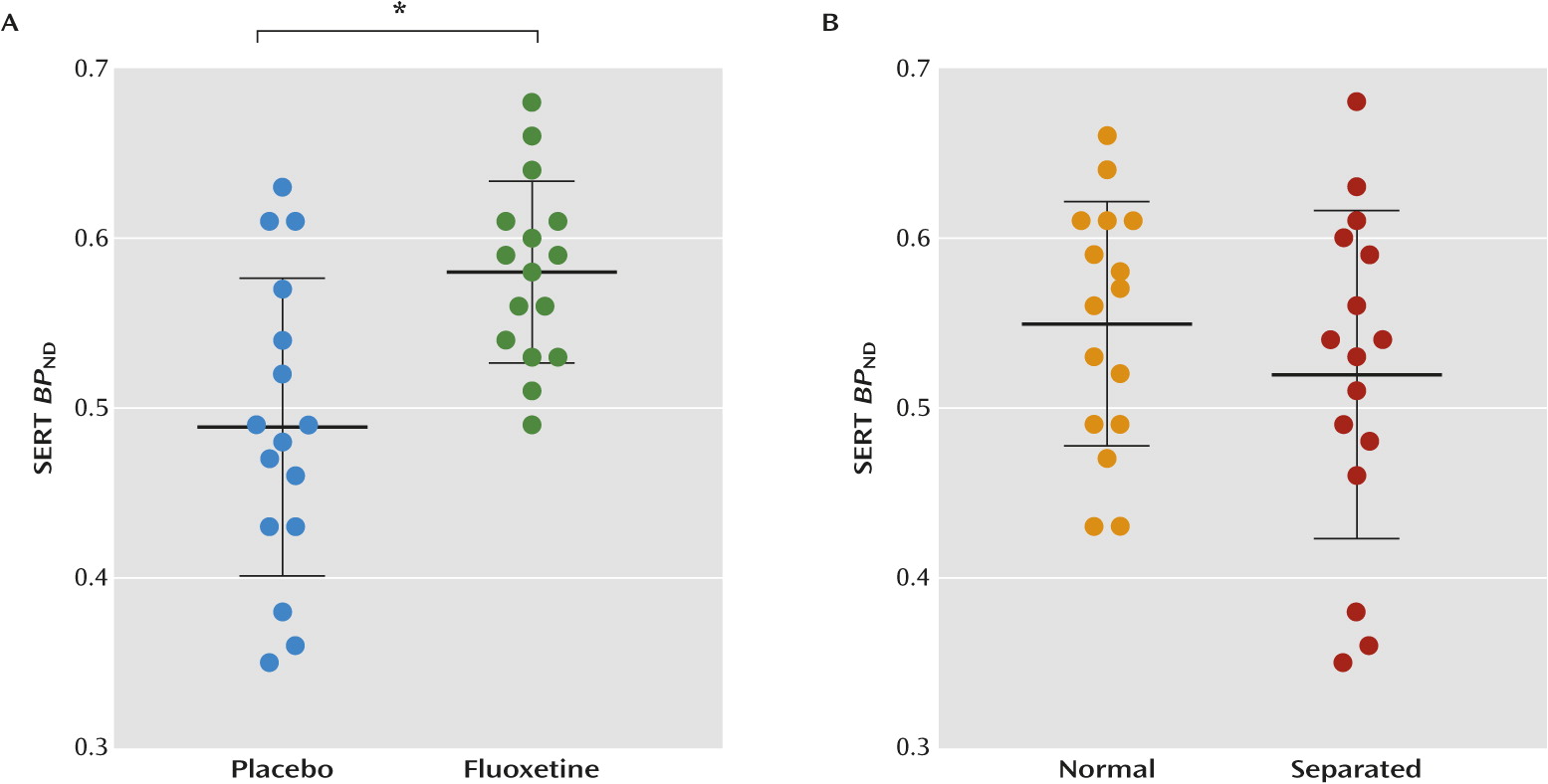
Fluoxetine upregulated SERT binding in the neocortex (+19%, F=12.8, df=1, 31; p<0.001×2=0.002;
Figure 2) and the hippocampus (+17%, F=6.6, df=1, 31; p<0.016×2=0.032; see Figure S3 in the online
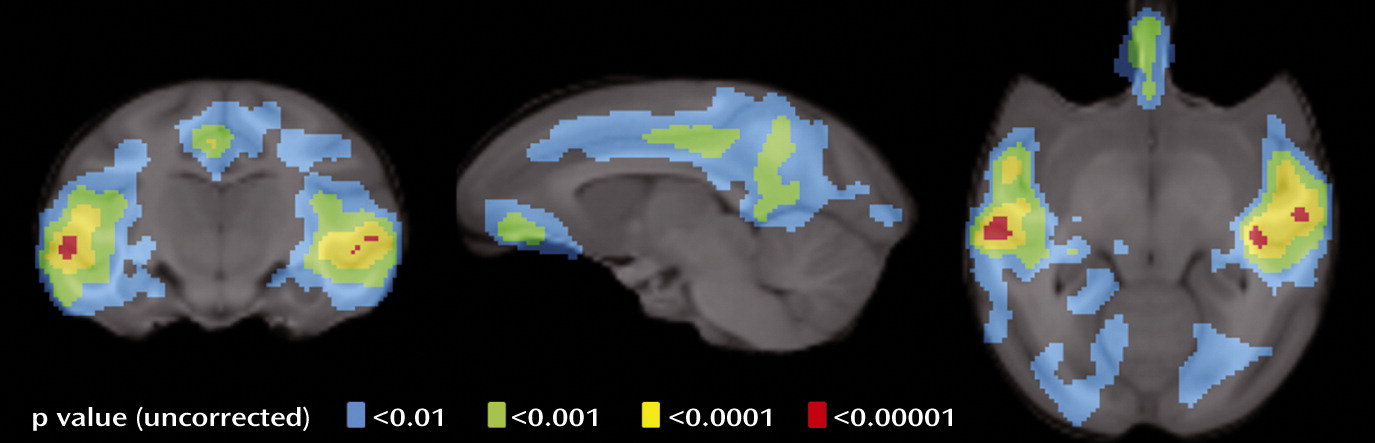
data supplement). Whole-brain voxel-wise analysis revealed that fluoxetine’s effects on cortical binding were localized to the lateral temporal, cingulate, and orbitofrontal cortices (
Figure 3). However, only the lateral temporal and posterior cingulate survived multiple comparisons at the voxel level after family-wise error correction (see Table S2 in the online
data supplement). Maternal separation had no significant effect on SERT binding. Two-way ANOVA analysis also revealed no statistically significant rearing-by-treatment interaction in either the hippocampus or neocortex.
Kinetic modeling essentially calculates the area under the time activity curves from time zero to infinity. Without a clear identification of the time of peak uptake and rate of washout (slope) prior to the end of the scan, the extrapolated area to infinity is vulnerable to error. Our scan period of 120 minutes was sufficient to calculate binding potential for both the neocortex and hippocampus, because both of these regions had early peak uptake and fast washout (see Figure S4, panel A, in the online
data supplement). However, we could not reliably quantify binding potential in the raphe because of its late time of peak uptake (consistent with its high SERT density), its slow washout from the brain, and its relatively high noise at later scan times. In fact, in some animals, radioactivity continued to rise in the raphe for the entire 120-minute scan (see Figure S4, panel A, in the online
data supplement); that is, we could not clearly identify the time of peak uptake. Such rising time-activity curves seemed randomly distributed in all four groups of monkeys. For these reasons, we excluded the raphe from our analysis of [
11C]DASB binding.
5-HT1A Receptor
In contrast to [
11C]DASB, [
11C](
R)-RWAY showed a time-activity curve in the raphe with a clearly defined time of peak uptake and rate of washout (see Figure S4, panel B, in the online
data supplement). Thus, all three regions (hippocampus, neocortex, and raphe) were analyzed for [
11C](
R)-RWAY.
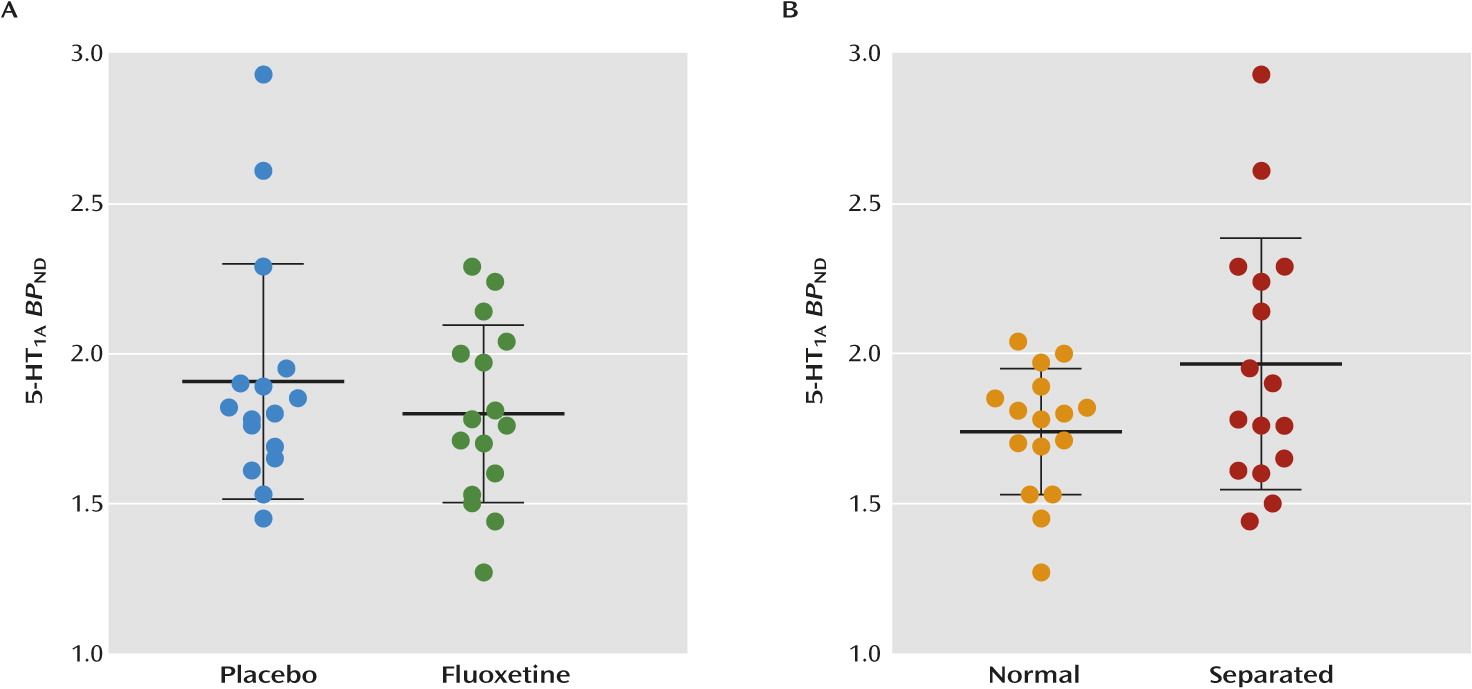
Neither fluoxetine nor maternal separation had a statistically significant effect on 5-HT
1A receptor binding (
Figure 4). Although 5-HT
1A receptor binding in the raphe was increased by 23% in maternally separated monkeys, this finding did not survive the statistical correction for the three regions examined (F=5.1, df=1, 29; p<0.03×3=0.09). Furthermore, no statistically significant effect was observed for each individual variable or any of the interactions using voxel-wise whole-brain analysis.
Social Behavior
Eight behaviors expressed in a social context (see Table S3 in the online
data supplement) were examined for effects of drug (i.e., fluoxetine compared with placebo), rearing, and period (i.e., whether animals were observed “during treatment” or “after treatment” with either fluoxetine or placebo). None of the behavioral effects of drug or rearing were statistically significant after correcting for multiple comparisons. However, overly liberal, uncorrected thresholds generated results that could be pursued in future studies. Namely,
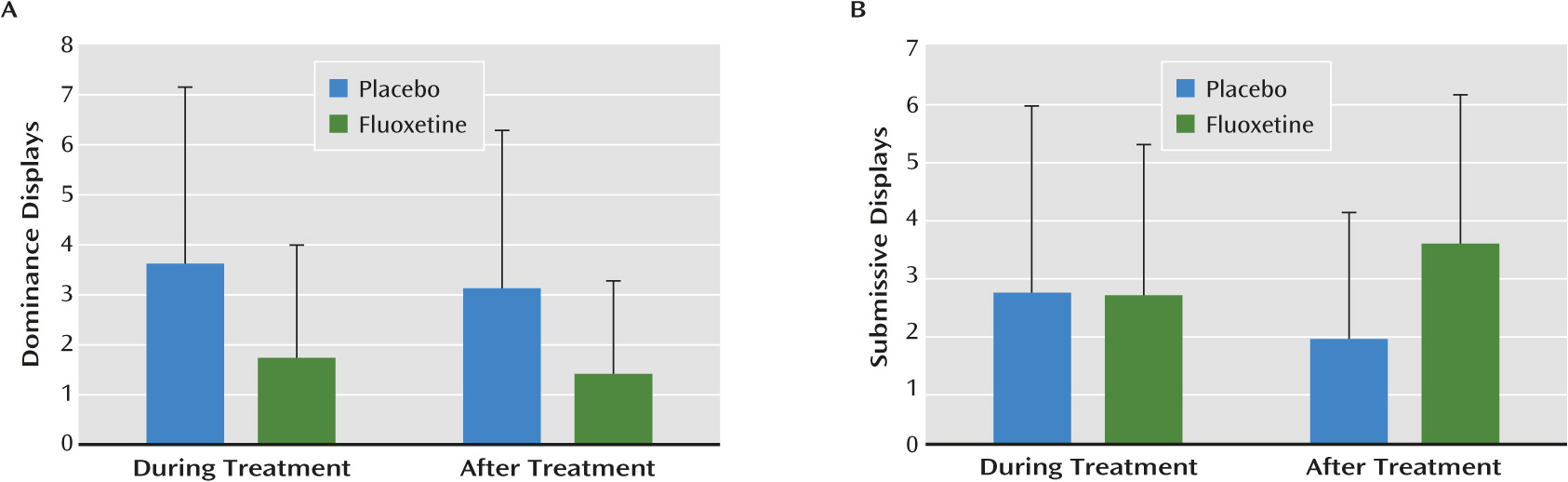
fluoxetine reduced dominance displays both during and after the treatment period in both rearing groups (F=4.75, df=1, 28; p<0.038×8=0.30). In addition, a drug-by-period interaction was observed for submissive displays (F=4.22, df=1, 28; p<0.049×8=0.39), reflecting between-group differences after, but not during, fluoxetine treatment (
Figure 5). Finally, we observed main effects of both period and rearing (independent of fluoxetine treatment) on other behaviors. Period effects were observed for locomotion (F=5.95, df=1, 28; p<0.02×8=0.16), passivity (F=18.01, df=1, 28; p<0.0002×8=0.002), and affiliative behaviors (F=22.35, df=1, 28; p<0.00006×8=0.0005). Only the period-related increase in passivity and decrease in affiliative behavior survived the statistical correction for multiple comparisons. The causes of these period-related effects are unknown but could reflect developmental changes, as the animals were almost 1 year older after treatment than during treatment.
Rearing effects were observed for locomotion (F=4.26, df=1, 28; p<0.048×8=0.38), stereotypy (F=7.01, df=1, 28; p<0.013×8=0.10), and bark frequency (F=5.04, df=1, 28; p<0.03×8=0.24). These rearing effects were unaffected by fluoxetine and persisted across both testing periods. However, as with the effects of treatment, none of the effects of rearing survived corrected statistical thresholds.
Discussion
This study examined the effects of long-term prepubertal fluoxetine administration on serotonergic neurotransmission in young adult monkeys (4.7 years old) that were either maternally separated or normally reared from birth.
Fluoxetine persistently upregulated [11C]DASB binding in the neocortex and hippocampus, regardless of rearing and more than 1.5 years after drug cessation. Whole-brain voxel-wise analysis revealed that fluoxetine had a statistically significant effect in the lateral temporal, cingulate, and orbitofrontal cortices. Interestingly, maternal separation at birth, which had previously been shown to induce lasting behavioral abnormalities in monkeys (
17), had no significant effect by itself on SERT binding in either the neocortex or hippocampus in this cohort of monkeys; this negative finding is tempered by the small number of placebo-exposed monkeys in each of the two rearing conditions.
With regard to the 5-HT1A receptor, fluoxetine had no significant effect in the neocortex, hippocampus, or raphe. While maternal separation increased 5-HT1A receptor binding in the raphe by 23% compared with normal rearing, this finding did not reach significance after correcting for multiple comparisons across the three regions (p=0.03×3=0.09). No rearing-by-treatment interaction was observed in any region for either SERT or 5-HT1A receptor. Taken together, our results demonstrate that long-term prepubertal fluoxetine treatment altered a key serotoninergic marker (SERT) into young adulthood, regardless of rearing.
Behavioral Effects of Adverse Rearing and Fluoxetine Treatment
A number of behavioral and neuroendocrine abnormalities have been demonstrated in maternally separated monkeys (
17,
30). Furthermore, previous studies with the current cohort, conducted prior to fluoxetine exposure, found that the adversely reared monkeys displayed several abnormal behaviors or responses, including increased fear-potentiated startle, increased consummatory behavior, increased cortisol secretion, altered locomotor activity, and altered circadian patterns (
31,
32). However, these rearing-related differences diminished as the animals aged. In the present study, no statistically robust rearing effects were found in either social behavior or other behaviors not reported here, including potentiated startle and cortisol. It is unclear whether the failure to find effects of rearing in adult monkeys was the result of habituation to repeated testing, repeated exposure to ketamine for routine handling, low statistical power (only eight monkeys in the two placebo cells), or normalization as a result of aging.
Just as differential rearing for the first 6 months after birth had no consistent effects on behavior, fluoxetine administered from age 2 to 3 years had no statistically significant effect on behavior. However, this negative finding must be tempered by the small sample size and relative high variability in the behavioral measures. For example, displays of dominance and submissiveness showed the greatest response to fluoxetine, with an effect size of 0.8, an effect considered to be medium-to-large in most behavioral studies. Nevertheless, using appropriate statistical criteria (two-tailed alpha=0.05 and power=0.8), an adequately powered study testing for an effect size of this magnitude requires 104 monkeys divided among four groups. Our study had only 32. Nevertheless, our cohort was unique and valuable, thereby justifying exploratory analyses to identify potential findings to be pursued either in a more focused way or in a much larger study.
Effects of SSRIs on 5-HT Neurotransmission in Rodents
Although the effects of long-term SSRI administration in early life have not been investigated previously in monkeys, rodent studies found that SSRIs had long-term effects on both neurochemistry and behavior that persisted into adulthood (
33,
34). Of direct relevance to the findings presented here, two previous rat studies (
5,
6) found that fluoxetine upregulated SERT in cortical regions by ∼20% when administered during the juvenile period, but not during adulthood. Furthermore, fluoxetine exposure during early life—but not adulthood—produced delayed, persistent perturbations of emotional behaviors similar to those seen in mice lacking SERT (
35,
36). A rat study (
34) found that fluoxetine increased some depressive-like behaviors but decreased others, with varying effects in immature and mature rodents. Biochemical studies of the effects of SSRIs on SERT and 5-HT
1A receptors in rats achieved varied and inconsistent results; various studies (
37–
40) have noted increases, decreases, or no changes in these markers, possibly a result of differences in strain, age, sex, and genetic polymorphisms.
Effects of Maternal Separation in Monkeys
Previous PET studies of monkeys with a history of maternal separation reported disparate effects on SERT and 5-HT
1A receptors (
18,
41). The present study found that maternal separation did not affect SERT density in the neocortex or hippocampus, but excluded the raphe for technical reasons. Two previous PET studies that used [
11C]DASB found that maternal separation either decreased SERT binding (
18) or had no effect (
42). Like the present study, the previous negative report scanned older monkeys (age 6.5 years in the study by Jedema et al. [
42] and age 4.7 years in the present study) than the one positive study (age 3 years). Thus, it is possible that any putative effects of maternal separation on SERT binding may normalize by 5–6 years of age. Notably, the timing of previous studies—between ages 3 and 6 years—bridges the pubertal transition in rhesus monkeys, which typically occurs at approximately 4 years of age.
Regarding 5-HT
1A receptors, the only previous study (
41) found that maternal separation increased binding in the dorso-medial prefrontal cortex of female, but not male, rhesus monkeys. However, this finding was likely not statistically significant after correcting for multiple comparisons, i.e., the study included multiple regions and three different measures of receptor binding (binding potential, density, and dissociation constant). The values of 5-HT
1A receptor binding potential in the present study differed from those in our previous publication (
27) because our new camera has higher resolution than the old one. The resolution of the camera used in the present study (Siemens microPET Focus 220) is ∼1.6 mm full width at half maximum, whereas that of the old camera (GE Advance) was ∼7 mm full width at half maximum. The higher resolution explains why binding potential values for a small region like the raphe are higher in the present study than in the previous publication (
27). In contrast, and as expected, resolution had little effect on large regions like the neocortex.
Advantages and Limitations
This prospective study in monkeys had the major advantage of examining the long-term prepubertal effects of fluoxetine in a well-controlled experimentally determined environmental setting. However, given the small sample size of only eight monkeys per group, the study has limited statistical power, making it vulnerable to both false positive and false negative results. To minimize false positives, we used stringent statistical analyses with both the Bonferroni correction for multiple measures as well as correction for multiple regions in our PET data. Nevertheless, our study was vulnerable to false negatives, which may have occurred, for example, with regard to the effect of maternal separation on 5-HT1A receptors in the raphe or of either separation or fluoxetine on behavior.
A second limitation was that our PET study looked at only one time point after fluoxetine administration. However, two previous studies in rats showed that fluoxetine has age-dependent effects and persistently upregulated SERT by ∼20%, primarily in cortical regions, when administered during the juvenile period but not in adulthood (
5,
6). A third limitation was that our PET study used ketamine to initially immobilize the monkeys; ketamine has widespread effects on glutamatergic transmission and induces rapid antidepressant effects in humans (
43). To minimize the effects of ketamine, we did not inject the radioligands until at least 120 minutes after the ketamine injection. Furthermore, while ketamine was used in all monkeys, including those receiving placebo, SERT upregulation was observed only in the fluoxetine group, not in the placebo group. A fourth limitation was that this and all previous PET studies used antagonist radioligands for the 5-HT
1A receptor. Antagonists do not discriminate between the active and inactive states of G-protein coupled receptors, including the 5-HT
1A receptor. In neuropsychiatric disorders, the active (i.e., agonist-preferring) state of the receptor might be more affected; thus, it would be useful to investigate this issue using agonist radioligands in the future.
Implications
Readers should note that many findings from behavioral and biochemical studies in monkeys and other animals are not replicated in humans. Accordingly, this study cannot directly address the safety or efficacy of SSRIs in children and adolescents with psychiatric disorders. Nevertheless, we provide guidance below on which of our findings may or may not parallel those in humans.
Most notably, this study was not designed to establish whether SSRIs are effective in treating children or adolescents with psychiatric disorders. First, the sample size was too small to assess the efficacy of fluoxetine on the behavioral effects of maternal separation in monkeys. Second, this animal model of maternal separation has never been validated as a measure of drug efficacy in humans.
In terms of potentially harmful effects associated with SSRIs, we have no evidence that the persistent upregulation of SERT observed here was either harmful or beneficial to the monkeys. In light of the known plasticity of the serotonergic system, we suspect that upregulation of SERT may have been part of several compensatory mechanisms to normalize 5-HT transmission in the brain. Because the human brain is thought to have similar homeostatic mechanisms, chronic SSRI use during development may persistently upregulate SERT in humans. In fact, we designed this study in monkeys to mimic fluoxetine treatment in a pediatric population. For example, the plasma concentrations of fluoxetine and norfluoxetine in monkeys were similar to those in children (see Figure S1 in the online
data supplement); the duration of administration (1 year in monkeys, equivalent to approximately 4 years in humans) occurs in clinical practice; and the drug had a similar, albeit diminished, pharmacodynamic effect on 5-HT turnover in monkeys as in humans, indirectly assessed as the concentration of a 5-HT metabolite in CSF (see Figure S2 in the online
data supplement). In addition, the washout period of more than 1 year resulted in no direct drug effect in the brain. In fact, any residual drug, if present, would have had the opposite effect—i.e., an apparent downregulation of SERT by competing with binding of the radioligand.
The only way to know definitively whether SSRIs persistently upregulate SERT in humans would be to study our species. Based on our results, it appears that such PET research studies, which are commonly performed in adults, may well be justified. To our knowledge, no PET study has examined the long-term effects of antidepressants on the serotonergic system in the adult human brain. It should be noted, however, that our results apply to the administration of SSRIs only during the prepubertal period, a time when the brain may have special developmental sensitivities. Furthermore, ethical concerns about radiation exposure to children, especially for an age-matched healthy comparison group, would likely preclude such a PET study. Nevertheless, given the robust, persistent effect of SERT upregulation observed in monkeys, it is possible that such changes would similarly be observed in children exposed to SSRIs. However, we do not know whether such changes are restricted to a particular developmental period; for instance, such persistent changes may or may not occur when SSRIs are administered in adulthood.
Conclusions
This study was to our knowledge the first in nonhuman primates to demonstrate that an antidepressant administered during development has long-lasting effects in the primate brain. The persistent SERT upregulation identified by the present study was a substantial, robust effect—particularly for a PET study conducted with such a limited sample size—and survived stringent statistical analyses both at the regional and voxel levels. Specifically, 2-year-old monkeys receiving fluoxetine, regardless of rearing, had persistently upregulated SERT binding 1.5 years after drug discontinuation. Implications regarding the efficacy or potential adverse effects of SSRIs in patients cannot be directly drawn from this study. Its purpose was to investigate the effect of SSRIs on brain development in nonhuman primates using an experimental approach that permitted the random assignment of long-term SSRI treatment or placebo. In contrast, human studies are necessarily confounded by the administration of SSRIs only to children with mental disorders, which themselves could affect brain development.
Acknowledgments
The authors dedicate this paper to the memory of our colleague, Dr. James Winslow, who was one of the leaders of this study before his untimely death in November 2010.
The authors acknowledge the support of the Intramural Research Program of the NIMH and thank Masanori Ichise, Masahiro Fujita, Sami S. Zoghbi, Paul Cumming, Allison Nugent, Kimberly J. Jenko, David A. Luckenbaugh, George M. Anderson, Alex Cummins, and the joint National Institutes of Health-Karolinska Institutet Doctoral Program in Neuroscience.