A dramatic rise in the incidence of depression occurs during adolescence, with approximately 14% of males and 28% of females experiencing a depressive episode by age 18 (
1). Longitudinal data indicate that an episode of depression during adolescence is a substantial risk factor for subsequent episodes, as well as for enduring social impairment (
2). Improved understanding of the mechanisms underlying adolescent depression may provide strategies to ameliorate such deleterious outcomes.
There is considerable evidence for structural brain alterations in cortico-limbic and striatal regions in adult depression, with meta-analyses reporting reductions in the volumes of the amygdala and hippocampus, of striatal regions including the caudate and putamen, and of prefrontal regions including the orbitofrontal cortex, anterior cingulate cortex, and dorsolateral prefrontal cortex (
3–
5). Structural abnormalities in similar regions have been implicated in adolescent depression, although the evidence base is smaller and the direction of effects is often different from those seen in adults (
6). Some studies have reported smaller hippocampus and amygdala volumes in depressed compared with healthy adolescents, and smaller hippocampal volumes in adolescents at risk for depression (
6), but other studies have reported no volumetric differences (
7,
8). The limited research on striatal volumes in adolescent depression is suggestive of reduced volume (
9). Findings regarding the prefrontal cortex have varied depending on the particular region, hemisphere, and sex of study participants (
6). One of the few investigations of cortical thickness in at-risk adolescents (
10) reported thinner cortices in the right lateral and left medial prefrontal regions, although some other regions showed increased thickness.
One limitation of the existing research on brain structure in adolescent depression is that all studies have used cross-sectional measurements of brain structure, which is problematic given normal maturational brain changes during adolescence (
11) and the fact that etiological and pathophysiological processes may differ depending on the stage of adolescence (
12). Indeed, emerging research suggests that attenuation of the normative pattern of prefrontal cortical thinning during adolescence is related to poorer emotional and cognitive functioning (
13). Longitudinal measures of brain development are therefore needed to clarify the nature of brain structural alterations in adolescent depression.
Our aim in this study was to investigate whether the development of limbic and striatal volumes and prefrontal cortical thickness from early to mid-adolescence was associated with the onset of depressive disorder. We hypothesized that abnormal developmental trajectories of all regions would be associated with depression onset. Specifically, attenuated thinning of prefrontal regions was hypothesized to be associated with onset of depression. While the lack of previous developmental work focusing on limbic and striatal regions did not allow more definitive directional hypotheses for these regions, previous cross-sectional findings would suggest that reduced growth across time would be associated with depression onset.
Method
Participants and Procedure
Participants were a community sample of 86 adolescents (41 of them female) recruited from schools across Melbourne, Australia, as part of a larger longitudinal cohort study (the Orygen Adolescent Development Study). The aim of the broader study was to examine risk and protective factors for depression during adolescence. In order to maximize variability in such factors, 2,453 early adolescents were screened on key affective temperaments known to promote risk and resilience for psychopathology. A subsample of 415 adolescents was selected to participate in further assessments. Equal numbers of males and females were selected from the following ranges of scores on the effortful control and negative affectivity dimensions of the Early Adolescent Temperament Questionnaire–Revised (
14): 0–1, 1–2, 2–2.5, and >2.5 standard deviations above and below the mean, resulting in an oversampling of those with high and low temperamental risk for psychopathology. Exclusion criteria included current or past depressive, substance use, or eating disorders according to the Schedule for Affective Disorders and Schizophrenia for School-Age Children–Present and Lifetime Version (K-SADS-PL) (
15); chronic illness; language or learning disabilities; and use of medications known to affect nervous system functioning.
Adolescents were invited to participate in four waves of assessments spanning approximately ages 12 to 18. Of the selected 415 adolescents, 245 agreed to participate in at least the first wave of research and met the inclusion criteria. At each wave, the K-SADS-PL was administered to assess current and past DSM-IV axis I disorders. At baseline, parents also reported on their child’s prior medical and mental health problems. At waves 1 and 3, participants underwent structural MRI scans. Depressive, anxiety, and externalizing symptoms were assessed at wave 1 using the Center for Epidemiologic Studies Depression Scale (
16), the Beck Anxiety Inventory (
17), and the externalizing scale of the Child Behavior Checklist (
18). Self-report versions of the former two questionnaires were used, whereas parent report was obtained for externalizing symptoms. At wave 1, intelligence was assessed by a short form of the WISC-IV (
19), handedness with the Edinburgh Handedness Inventory (
20), and pubertal status (Tanner stage) with the Pubertal Development Scale (
21). Socioeconomic status was estimated using the Australian National University Four (ANU4) Scale (
22). The final sample of 86 represented those participants who consented to participate in brain imaging at waves 1 and 3 and diagnostic interviews at waves 1, 3, and 4 (
Table 1).
During the follow-up period (ages 12 to 18), 30 adolescents (20 of them female) experienced a first onset depressive disorder. The mean number of episodes was 1.77 (SD=0.97), the mean episode duration was 21.43 weeks (SD=23.56), and the mean age at onset was 15.81 years (SD=2.11). Of the 56 adolescents who did not experience a depressive disorder during the follow-up period (the comparison group), 16 experienced another form of psychopathology (
Table 2).
At each wave, written informed consent was obtained from all adolescents and their parent or guardian in accordance with the guidelines from local research and ethics committees.
Neuroimaging
Acquisition and preprocessing.
At wave 1, MRI scans were performed on a 3-T GE scanner (repetition time=36 ms, echo time=9 ms, flip angle=35°, field of view=20 cm2) to obtain 124 T1-weighted contiguous slices (voxel dimensions=0.4883×0.4883×1.5 mm). Wave 3 scans were conducted on a 3-T Siemens scanner (repetition time=1900 ms, echo time=2.24 ms; flip angle=9°, field of view=23 cm2), producing 176 T1-weighted contiguous 0.9 mm thick slices (voxel dimensions=0.9 mm3).
The stability of image acquisition in longitudinal or multisite studies may be compromised in several ways, including instrument-related differences between sites and instrument or software upgrades within sites (
23). We took steps to address errors that are known to result from multisite or longitudinal scanning, including postprocessing and examination of interscanner bias via an interscanner reliability study (see the
data supplement that accompanies the online edition of this article). Based on small differences between regional volume and thickness measures between scanner platforms, a correction factor was applied to structural measures from the wave 1 scans after segmentation. Although application of the correction factor had no effect on results, it was performed to improve interpretation.
Measurement of volume and thickness for regions of interest.
Hippocampus, amygdala, and striatal (caudate, putamen, pallidum) volumes and mean thickness of prefrontal regions were estimated at waves 1 and 3 using the longitudinal stream in FreeSurfer, version 5.1 (
http://surfer.nmr.mgh.harvard.edu/fswiki/LongitudinalProcessing), which has been used extensively in child and adolescent populations and has been validated for use in children as young as age 4 (
24). This automated procedure involves the assignment of a neuroanatomical label to each voxel in an MRI volume based on probabilistic information estimated automatically from a manually labeled training set. Customized anterior cingulate, orbitofrontal, dorsolateral, and ventrolateral prefrontal cortex regions were created (see the online
data supplement).
All segmentations were visually inspected by trained researchers blind to participant characteristics. Corrections to the gray matter-white matter surface were made to the majority of images, and (of the 117 longitudinal data sets) 15 images were discarded because of excessive artifact or poor segmentation. Whole brain volume was calculated at both waves as the total of all gray and white matter from the FreeSurfer segmentation. Average cortical thickness was calculated at both waves as the average thickness across all cortical regions, weighted by their surface area.
Volume and thickness measures (corrected for interscanner bias as described in the online
data supplement) were corrected for whole brain volume and average cortical thickness, respectively, using a covariance procedure (
25), and these corrected volume and thickness estimates were used in all subsequent analyses.
Attrition Analysis
There were no differences between the final sample (N=86) and both the screening sample (N=2,453) and the selected sample (N=415) on socioeconomic status, affective temperament scores at screening, or sex. In one-sample Kolmogorov-Smirnov tests, the final sample did not differ from the selected sample in terms of the distribution of affective temperament scores; that is, the distribution of affective temperament scores for the final sample was flatter than the normal distribution.
Data Analysis
Repeated-measures analyses of covariance (ANCOVAs) were performed to assess whether change in whole brain corrected regional volume and in thickness measures from early to mid-adolescence (ages 12 to 16) was associated with depression onset from early to late adolescence. Separate analyses were conducted for each region. For each analysis, the between-subject variables were group (depressed, comparison) and sex (male, female), and the within-subject variables were hemisphere (left, right), and time (early adolescence [age 12], mid-adolescence [age 16]). Depressive and anxiety symptoms at wave 1, socioeconomic status, time between imaging assessments, and Tanner stage were used as covariates. For any models containing significant group-by-time effects, follow-up tests were conducted on cross-sectional group differences in brain structure at early and mid-adolescence. For any analyses involving significant effects of group, ANCOVAs were repeated excluding participants who developed a depressive disorder before mid-adolescence (N=15), to test whether brain structural measures were a prospective predictor of depression onset during mid to late adolescence. Finally, for any models involving significant effects, Pearson’s correlations were computed to investigate whether there were any relationships between regional volume and thickness measures and age at first onset of depressive disorder. Spearman’s rank correlations were computed to investigate associations with duration of depressive illness and number of depressive episodes (as these variables were skewed).
Results
Demographic and clinical data for the two groups are provided in
Table 3. The adolescent depression group had a higher proportion of females and, on average, lower socioeconomic status, higher baseline depressive and anxiety symptoms, a longer interval between the two imaging assessments, and more advanced pubertal status than the comparison group. The depression group also had a higher proportion of comorbidities than the comparison group. Mean volume and thickness estimates at each wave and associations between all continuous variables are provided in the online
data supplement.
No significant correlations were found between measures of volume or thickness and clinical variables.
Hippocampus and Amygdala
Significant main and interaction effects were observed for all brain regions (
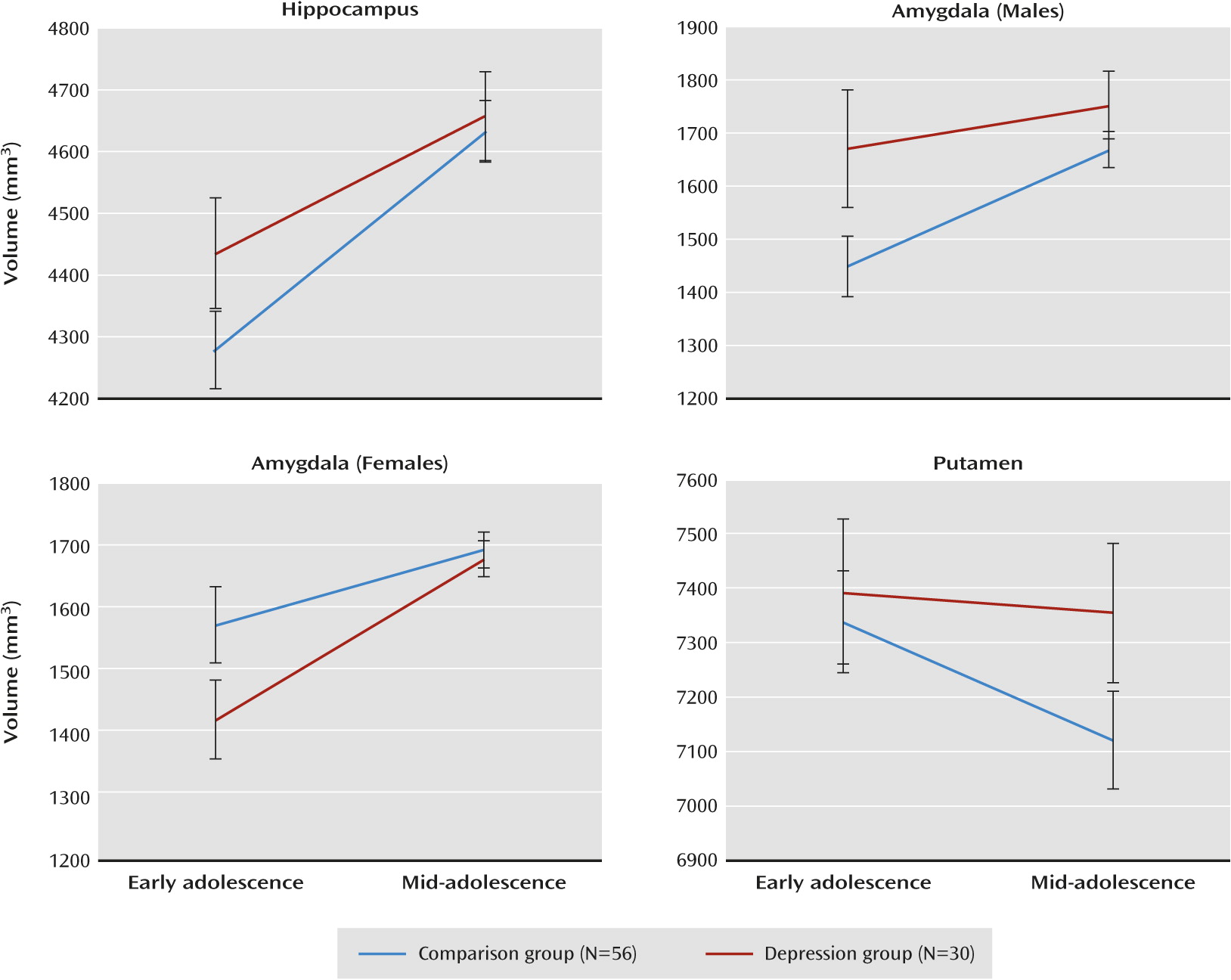
Table 4); nonsignificant effects are not reported for simplicity. Regarding the hippocampus, there were significant group-by-hemisphere and group-by-time effects. While there appeared to be an effect of larger hippocampal volumes in the depression group that was more pronounced for the left hemisphere (across time), the group effect was not significant for either the left or the right hemisphere.
Figure 1 displays the group-by-time effect and shows that hippocampal growth from ages 12 to 16 was greater for the comparison group as compared with the depression group. There were no effects of group on hippocampal volume in early or mid-adolescence. When the initial analysis was rerun excluding participants who developed a depressive disorder before mid-adolescence (N=15), the group-by-time interaction remained significant (F=11.32, df=1, 62, p=0.001).
Regarding the amygdala, there were significant effects of group by sex and group by sex by time.
Figure 1 shows that for females, increased growth of the amygdala from early to mid-adolescence was associated with onset of depressive disorder, whereas for males, reduced growth was associated with onset of depressive disorder. Follow-up tests showed that the group-by-time interaction approached significance for females only (p=0.056; males, p=0.107). There were no group differences in amygdala volumes in early or mid-adolescence for males or females. When the initial analysis was rerun excluding participants who developed a depressive disorder before mid-adolescence, the group-by-time-by-sex interaction remained significant (F=5.19, df=1, 62, p=0.026).
Striatal and Prefrontal Regions
For the putamen, there was a significant group-by-time effect. As shown in
Figure 1, volumetric reduction of the putamen from ages 12 to 16 was greater for the comparison group compared with the depression group. There were no group differences in putamen volumes at early or mid-adolescence. The group-by-time effect did not remain significant after excluding participants who developed a depressive disorder before mid-adolescence.
For the nucleus accumbens, there was a main effect of time and a significant group-by-sex interaction. The nucleus accumbens increased in size over time and (averaged across time) was smaller in the depression group compared with the comparison group in females only (p=0.008; males, p=0.664). The group-by-sex effect remained significant after excluding participants who developed a depressive disorder before mid-adolescence (F=7.00, df=1, 62, p=0.011). There was a significant effect of time for the dorsolateral prefrontal cortex, with significant thinning over time. There were no significant effects for any other striatal or prefrontal cortical region.
Discussion
To our knowledge, this is the first study to use a longitudinal design to assess whether structural development of the brain is associated with first onset of depressive disorder during adolescence. A high proportion (34%) of adolescents experienced a first episode of depressive disorder over the follow-up period, likely because of the sampling strategy. We found that an attenuation of the normative pattern of change in hippocampus, putamen, and amygdala volumes (the latter for males only) from ages 12 to 16 was associated with the onset of depressive disorder during adolescence. For females, we found that accelerated growth of the amygdala from ages 12 to 16 and smaller nucleus accumbens volume (across time) were associated with depression onset. Significant effects for the hippocampus, amygdala, and nucleus accumbens remained after excluding cases of depression onset prior to mid-adolescence, suggesting that abnormal development of these regions may predate onset of the disorder and thus represent a vulnerability factor. There were no associations between the development of prefrontal regions and depression.
Hippocampus and Amygdala
Although we found that patterns of hippocampal and amygdala growth from ages 12 to 16 predicted onset of depressive disorder, our findings were inconsistent with our hypothesis of a main effect of smaller hippocampal and amygdala volumes predicting disorder onset. While a number of studies have reported smaller volumes in adolescents with depression and those at risk for depression (e.g., reference
6), these studies used cross-sectional measures of brain structure, and group differences were averaged across participants spanning much broader age ranges (e.g., 12–20 years;
26). Furthermore, our finding of marked sex differences in the association between change in amygdala volumes and depression onset may help to explain some of the inconsistencies in the previous literature, given that some studies have used mixed-sex samples but have not examined sex differences (
27).
For both the hippocampus and the amygdala, associations held when considering only cases of depression with onset after the mid-adolescent imaging wave, suggesting that the patterns of development associated with depression onset may represent risk markers. Given that these structures have key roles in modulating endocrine stress systems (
28) and in emotional reactivity, learning, and memory (
29), our findings may indicate that disruptions to these processes are critical to the etiology of adolescent depression. Interestingly, the hippocampal developmental pattern in those at risk seemed to reflect an attenuation of the normative pattern of growth across adolescence (
11). This attenuation may reflect damage to hippocampal neurons over time or, alternatively, disruption to neurogenesis. Animal studies suggest that both could be triggered by stress-related hypothalamic-pituitary-adrenal (HPA) axis dysfunction (
30). This speculation is consistent with the demonstrated association between stressful life experiences and both the onset of depressive episodes (
31) and hippocampal volumes in young people (
32). However, genetic factors that might predispose certain patterns of hippocampal development cannot be discounted (
33).
Our amygdala findings may relate to sex differences in the normal pattern of amygdala growth across adolescence. Previous work has shown more rapid amygdala development in males compared with females (
34), a finding that was mirrored in the patterns of growth for the comparison group in the present study. It is thus possible that the observed sex effects were due to males and females being at different points in their amygdala growth trajectories during the period studied.
Our amygdala findings might also be explained by the interaction of gonadal hormones with the function of the HPA and monoaminergic systems, as suggested by animal studies that have shown environmental stressors to have opposing effects on the number of serotonin receptors in the male and female amygdala (
35). Genetic mechanisms may also account for our findings, as previous work shows genetic predictors of amygdala volume and affect-related functional connectivity (
36).
Prefrontal Cortex and Striatum
We found no associations between development of prefrontal cortical thickness and depression, despite other research finding associations between thinning of the prefrontal cortex across adolescence and depressive symptoms (
13). It is possible that alterations in prefrontal cortex development during adolescence is associated with risk for depression onset beyond the period captured in the present study.
We found significant associations between striatal development and depression onset. Specifically, a lack of the “normal” pattern of volumetric reduction over time in the putamen (
11) was associated with depression onset. Although other research has shown smaller putamen volumes in adults with depressive disorder (
37), in the only other study examining putamen volumes in adolescent depression, Matsuo et al. (
9) found no group differences. Our finding of a lack of putamen volume reduction in depressed adolescents may reflect a deficit in synaptic pruning or myelination, processes that have been attributed to both cortical and striatal gray matter development during adolescence (
11). It has been suggested that concurrent patterns of development in the putamen and frontal cortex may facilitate the maturation of cognitive and motor functions that rely on connections between these areas (
38), and hence abnormal putamen development in adolescent depression may be associated with deficits in such functions. Rodent research has shown an elimination of dopamine receptors across adolescence, a pattern that mirrors the normative pattern of volumetric development in humans (
39). Thus, the abnormalities in putamen development observed in depressed adolescents may be associated with altered reward function.
We found that smaller nucleus accumbens volumes predicted depressive disorder onset in females, with some evidence that this volume reduction may indicate vulnerability to depression. Although no studies to date have implicated alterations in nucleus accumbens volume in adolescent depressed patients, reduced nucleus accumbens volume has been associated with anhedonia in healthy individuals (
40). Given this finding, along with research indicating that the nucleus accumbens has a role in processing rewarding stimuli (
41), it is possible that smaller volumes might reflect reward-related dysfunction. That our finding was specific to females might also be consistent with research showing sex differences in nucleus accumbens dopaminergic function (
42).
Limitations and Future Directions
Several limitations to our study should be noted. First, the small numbers of male participants who developed a depressive disorder across the follow-up period may have reduced our study’s power to find significant effects. Second, the higher proportion of comorbid disorders in our depressed participants limits the inferences we can draw about the specificity of the findings to depression. Third, as noted, MRI acquisition was multisite and used different sequences. We did, however, take steps to measure and correct for any interscanner/sequence bias, and it is unlikely that depression interacted with scanner type in any systematic manner that would bias results. Fourth, it has been suggested that reliability for some FreeSurfer-delineated structures is relatively low; however, sample sizes of approximately 80 and above are suggested to be adequately powered to detect small to medium effect sizes (
43). Fifth, pubertal data were available only from the baseline assessment, so we cannot comment on how pubertal maturation might be associated with brain development and onset of depressive disorder. Sixth, we captured maturational change during a specific early to mid-adolescence period. It would be of interest to assess whether the abnormal developmental patterns associated with depression eventually normalize or continue to diverge. It would also be of interest to assess whether differences in brain structure exist earlier in childhood and therefore might represent very early neurobiological risk factors for later development of depression.
Conclusions
This study demonstrates for the first time links between the development of the amygdala, hippocampus, and striatum and onset of depressive disorder during adolescence. Our findings highlight the complex relationship between neurodevelopment, sex, and vulnerability to depression during adolescence and suggest that the relationship of subcortical volumes to depression onset may be best captured by measures of longitudinal change or development, at least for the period from early to mid-adolescence. These findings may have implications for early detection and interventions for individuals at risk for depression during adolescence.
Acknowledgments
Neuroimaging analysis was facilitated by the Neuropsychiatry Imaging Laboratory at the Melbourne Neuropsychiatry Centre. The authors thank the Brain Research Institute and Royal Children’s Hospital for support in acquiring the neuroimaging data.