Cross-sectional MRI studies have convincingly shown that brain volumes in schizophrenia patients are smaller than those in healthy subjects. Some of these abnormalities, such as changes in white matter volume and structure, are present before illness onset (
1,
2) and are most likely of a developmental (
3), possibly genetic (
4), nature and appear to be stable over time (
5). In contrast, other brain changes, such as reductions in gray matter volume, become more pronounced during the course of the illness (
6). Although several studies suggest that gray matter volume reductions are related to outcome (
7), psychosis (
8), relapses (
9), medication (
10), cannabis use (
11), and genetic liability (
12), the cause and nature of the progressive loss of gray matter are still unclear.
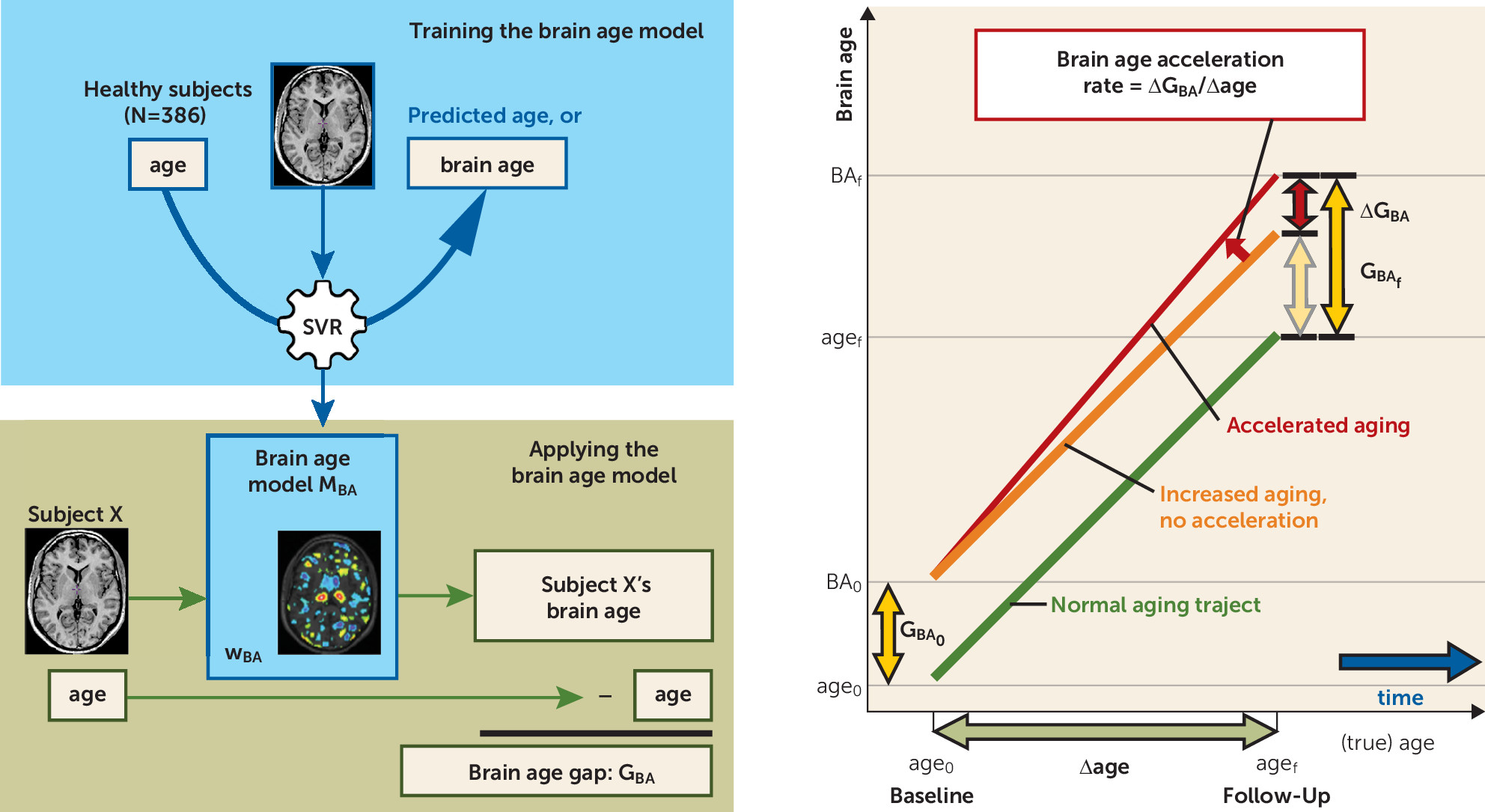
Indeed, despite the multitude of longitudinal neuroimaging studies, a basic question on the progressive brain loss in schizophrenia remains unaddressed: Does it reflect accelerated aging of the brain, or is it caused by a fundamentally different process? Here, we address this question by using a relatively new technique, support vector regression (
13), a supervised machine learning technique used to make predictions based on high-dimensional (image) data. A model can be trained to recognize patterns in brain tissue that are associated with aging. Using these patterns, the model then transforms, or aggregates, the high-dimensional image data of each individual into a predicted age, or brain age. Comparable techniques have successfully been applied to MRI scans leading to age predictions in adults (
14) and across the lifespan (
15), as well as development/maturation indices in children and young adults (
16–
19). The advantage of such techniques over univariate analyses is that they detect and use the coherence between voxels involved in aging and are capable of dealing with the large variation in brain structures between subjects.
The aging of the brain in schizophrenia patients, as estimated from their brain images, was found to be increased compared with the chronological age of the brain (
20). On average, this “brain age gap” estimate (
14) was 5.5 years. The results of such cross-sectional studies support the use of machine learning to study brain development in schizophrenia across the lifespan. However, longitudinal studies are required to capture the dynamic aspects of aging, truly measuring (abnormal) accelerations or decelerations in the aging of the brain.
To investigate whether the brains of schizophrenia patients age in an accelerated fashion, we measured brain age in a large longitudinal sample of healthy subjects and schizophrenia patients. An age prediction model was trained on the healthy subjects’ baseline image data. It was subsequently applied to the follow-up scans of the healthy subjects and the baseline and follow-up scans of the patients. Brain age gaps and accelerations were calculated and, for patients, related to duration of illness. In addition, a model separating schizophrenia patients from healthy subjects was built. Using these models, we were able to separate the effects of normal aging on the brain and those specific to schizophrenia.
Results
Brain Age Model: Accuracy, Validity, and Reliability
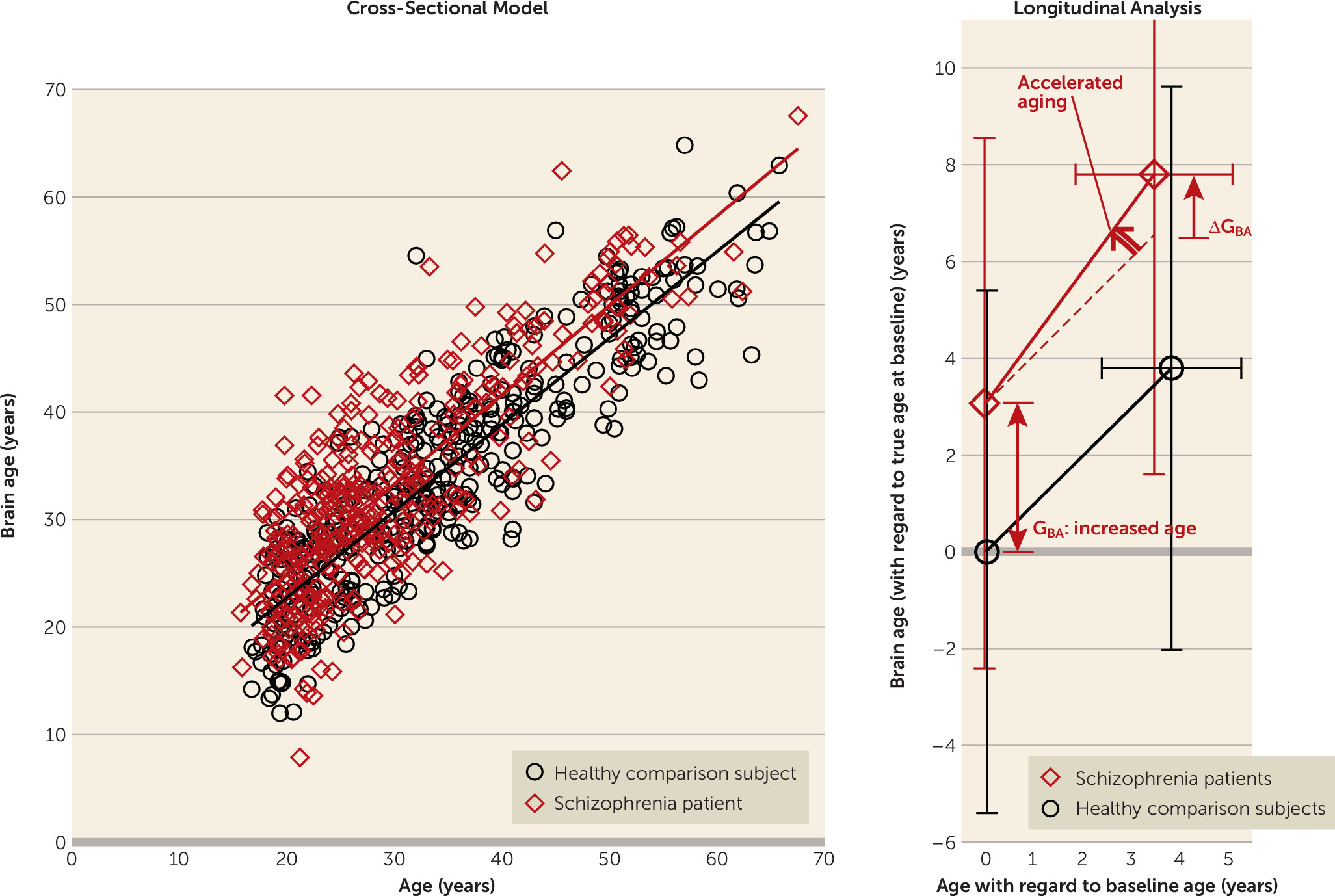
The brain age model explained almost 80% of the baseline age variance (R
2=0.79) in healthy subjects. The mean absolute error was 4.31 years. These values are comparable to earlier models (4.98 years (
14); 4.6 years (
20), R
2=0.83) (
Figure 3).
Mean brain age gap at baseline for the healthy subjects was, as expected, almost zero: GBA=−0.0017 years (SD=5.40) (less than 1 day).
Application of the brain age model to the 3-T images yielded a mean brain age gap of −0.18 years (SD=4.80) (mean absolute error=3.86 years) in healthy subjects and +5.59 years (SD=5.11) in schizophrenia patients.
When applied to the brain images of subjects who were scanned twice within 12 days, the brain age model yielded differences in brain age with a mean of 0.062 years (SD=1.516).
Applying a “reverse” brain age model, built on brain images of schizophrenia patients, yielded a brain age gap of −4.83 years for the healthy subjects (see the online data supplement).
Application of the Brain Age Model to Patient and Follow-Up Scans
In healthy subjects, the mean brain age gap at follow-up (on average 3.84 years later [SD=1.44]) was GBA=−0.045 years (SD=5.82) (16 days), not significantly different from zero. This shows that, on average, the aging of healthy subjects’ brains was consistent with their increasing chronological age during the interscan interval.
At baseline, the brain age of schizophrenia patients was significantly greater than their chronological age: GBA=3.36 years (SD=5.87) (N=341; t=10.73, p<0.001), indicating excessive aging of the brain at baseline.
At follow-up (on average 3.48 years later [SD=1.62]), the brain age of the patients had progressively increased by 4.72 years (SD=4.14), increasing the gap to G
BA=4.32 years (SD=6.20) (N=192; t=9.65, p<0.001); ΔG
BA=1.24 years (SD=3.81) (t=4.53, p<0.001). The aging thus accelerated at a rate of 4.72/3.48=1.36 years/year, or an additional 4 months in each year during the follow-up period (
Figure 3).
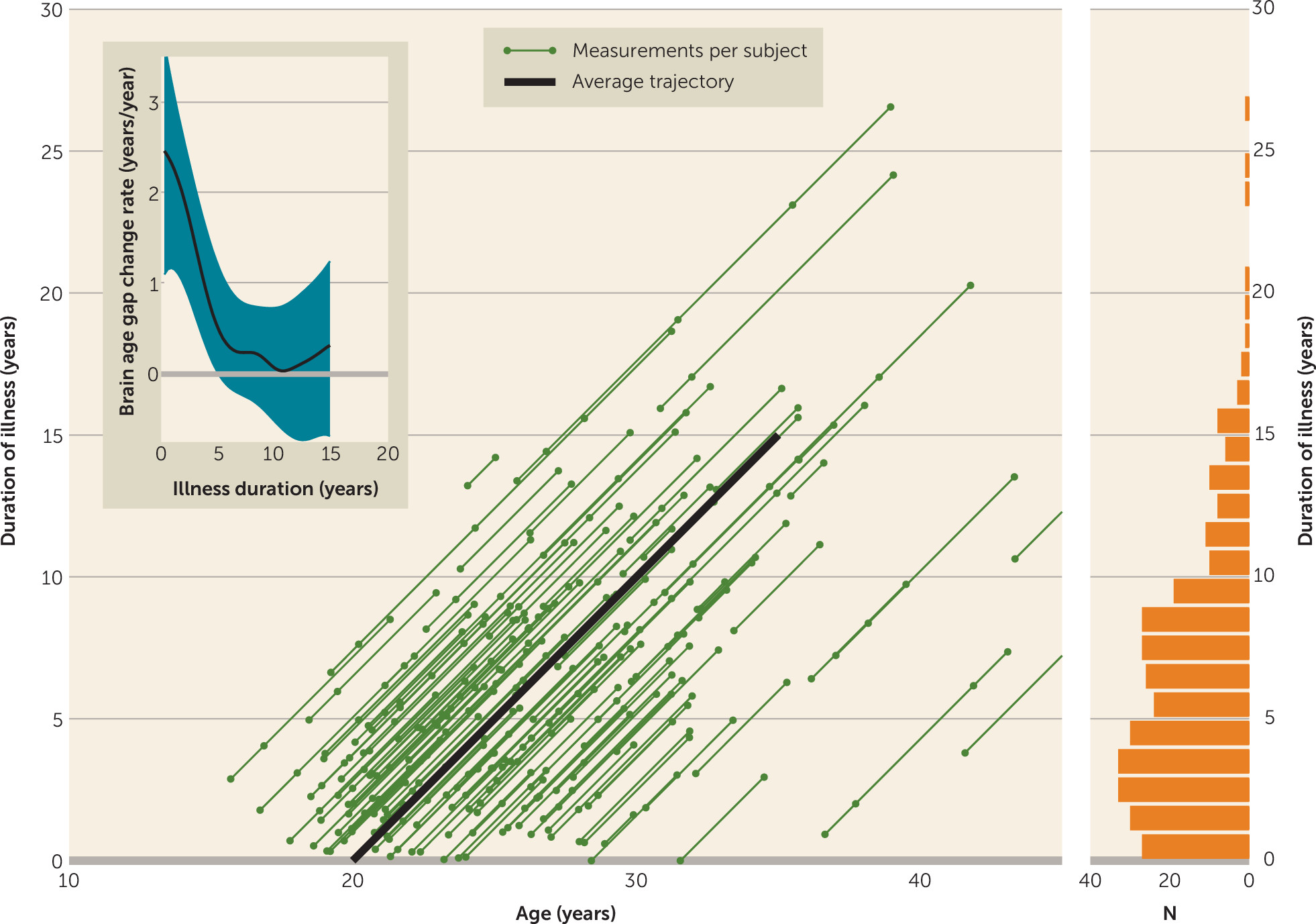
Brain Age Acceleration Along the Age and Duration-of-Illness Trajectory
Results of the locally weighted regression of ΔG
BA/Δage for the “average” patient’s age and duration-of-illness trajectory are shown in
Figure 2 (inset). Just after illness onset, the brain age gap starts growing by about 2.5 years/year—in other words, when the patient has become 1 year older (and thus has been ill for one more year) his or her brain age has become 3.5 years older. Subsequently, the acceleration rate rapidly slows over the first few years of the illness. After about 5 years, the acceleration is no longer significant: the brain age gap stays constant.
Separation of Healthy Subjects and Schizophrenia Patients: Comparisons in Gray Matter Density Space
The schizophrenia model separated patients and healthy subjects with an accuracy of 68.6%. From baseline to follow-up, the size of the gray matter density component associated with the brain age gap (G
BA), the brain age fingerprint, increased by 0.151 (t=2.35, df=191, p<0.01) for schizophrenia patients as compared with healthy subjects (see Table S1 in the
online data supplement). From baseline to follow-up, the length of the brain-age-corrected gray matter density component associated with the schizophrenia gap (G
SZ), the schizophrenia fingerprint, changed by 0.132 (t=0.77, df=191, n.s.) in the direction of “more schizophrenia” for patients as compared with controls. Although about as large as the change of the G
BA-associated fingerprint, the change in the schizophrenia fingerprint was not significant because of the large standard deviation of the changes, which in turn was related to a large increase in the standard deviation of the size of the patient’s fingerprint at follow-up, from 0.59 to 1.87 (F=3.17, p<0.001).
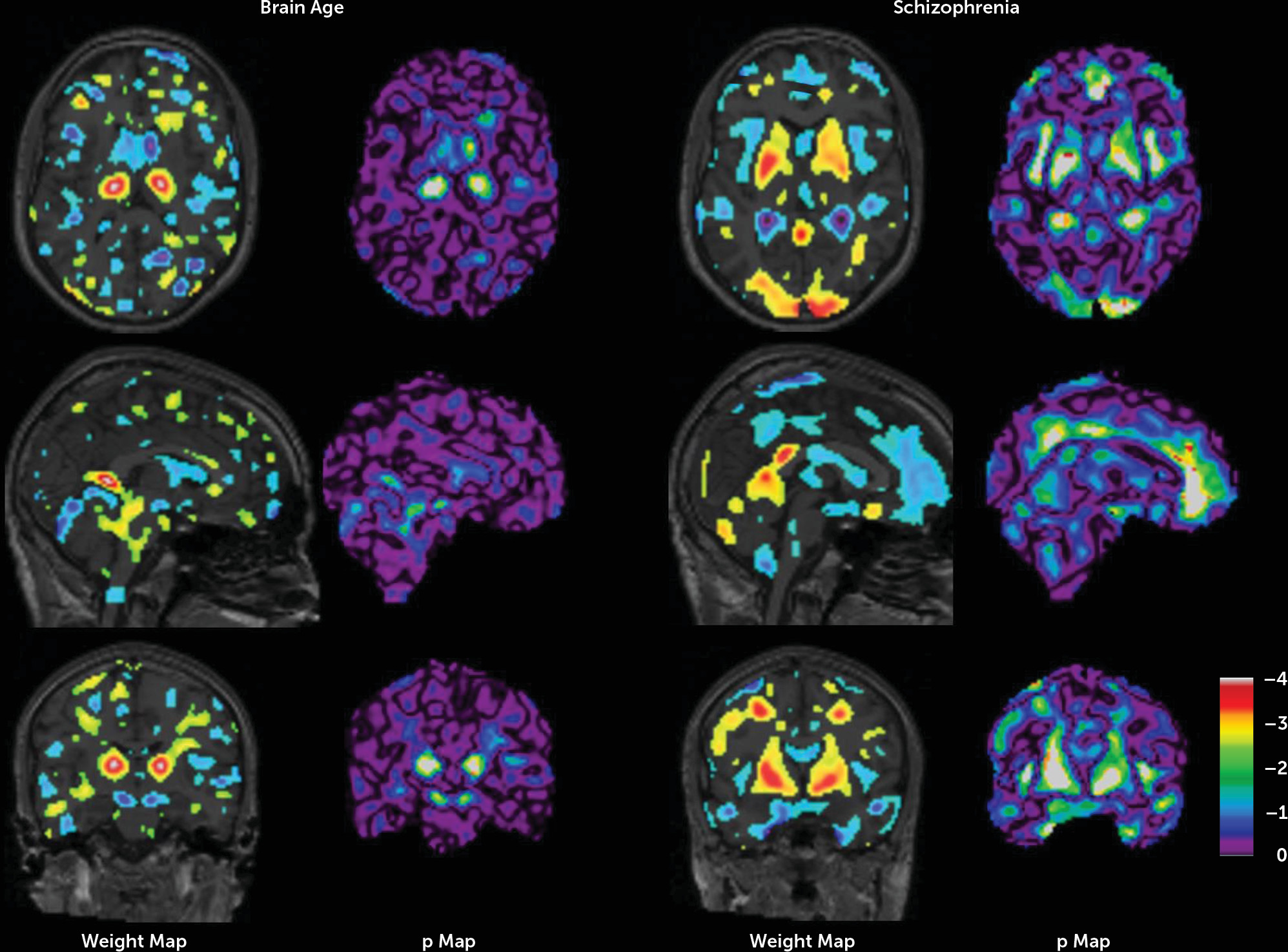
Figure 4 shows the weight maps of the models and their significance.
Applying a GBA=1.55-year threshold value, GBA can be used to separate patients and healthy subjects with an accuracy of 60.2% (a sensitivity of 59.5% and a specificity of 60.9%). Training a two-feature support vector machine on the (GBA,GSZ) fingerprints resulted in a schizophrenia classification model with 71.5% accuracy (see Figure S4 in the data supplement).
Relationship With Clinical Parameters
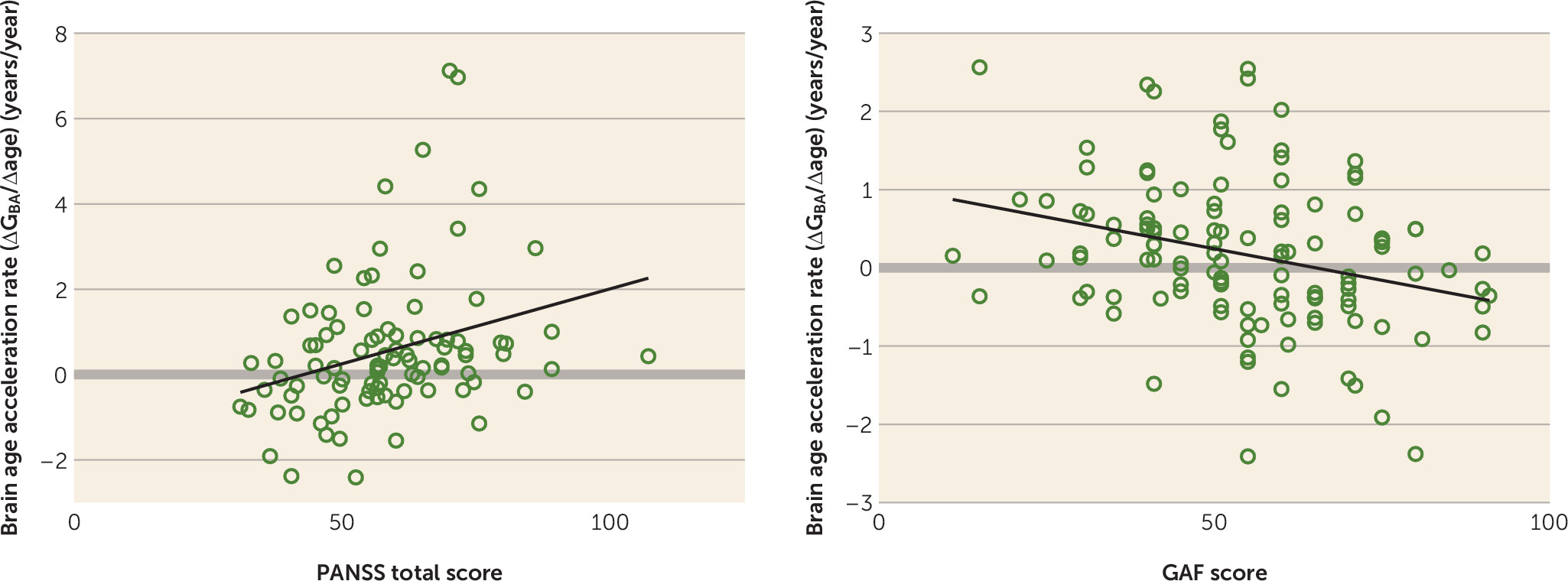
At follow-up, brain age gap was significantly negatively associated with GAF score (
Figure 5; see also Table S3 in the
data supplement) and positively with antipsychotic dosage (see Table S3). Brain age acceleration rate was negatively associated with GAF score and positively with PANSS total score at follow-up, and positively with number of hospitalizations, duration of hospitalization, and cumulative antipsychotic intake. The latter was also significantly associated with schizophrenia gap acceleration rate.
Discussion
This longitudinal study in 727 subjects with 1,197 scans is, to our knowledge, the first to show that some of the frequently and consistently reported progressive changes in the gray matter morphology of schizophrenia patients (
6) resemble, and possibly reflect, an accelerated aging process that is related to outcome. The remaining part of the progressive changes in patients is qualitatively different from the brain changes observed in healthy subjects over time, and possibly reflects individual variation related to the illness and medication use.
At baseline, brain age in schizophrenia patients was 3.36 years greater than chronological age, replicating earlier results (
20) (but see “Methodological Considerations” in the
data supplement for a discussion of the possible influence of methodology on our finding of a brain age gap smaller than that reported in reference
20). This means that at around 4 years of illness (when baseline scans were made in this study) brain morphology in schizophrenia patients is similar to that of healthy subjects who are more than 3 years older. Despite this already considerable age gap at baseline, the brain morphology of the schizophrenia patients showed (further) accelerated aging over the 3.5-year follow-up period. Specifically, while the pattern of brain aging in the healthy subjects developed in line with the increase in their chronological age, in the schizophrenia patients this pattern progressed at an augmented pace of 1.36 years/year: an additional 4 months in each year during the follow-up period.
We also found differences between patients and healthy subjects in gray matter change that were qualitatively different from those involved in aging. The “schizophrenia gap”—the aggregate of these differences—was present at baseline and widened during the interval. Interestingly, the variance increased significantly over the interscan interval, rendering the widening of the schizophrenia gap over time nonsignificant. This suggests that these illness-specific changes are more related to the individual course of the disease. Indeed, an association (albeit a weak one) with outcome was found, while differences in medication played a much more significant role in acceleration of the schizophrenia gap than of the brain age gap (see Table S3 in the data supplement). Conversely, the relative interindividual stability of the accelerated brain aging in the schizophrenia patients suggests that it is due to shared factors underlying the disease.
Healthy subjects displayed considerable variability in changes in their brain age gaps during the measurement interval as well. While these changes were on average zero, their standard deviation was 2.7 years. A large part of these changes (2.2 years, as determined from repeated scans) can be attributed to real structural aging of the brain. The nature and cause of these changes are unknown, but they could be of genetic origin or be related to cognitive functioning (
36,
37) or to differences in lifestyles or physical (
29,
38) and mental (
39) activity.
The accelerated aging of the brain in schizophrenia takes place predominantly during the first years after disease onset. After the first year of illness, the brain age gap had increased by about 2 years. Over the first 5 years of follow-up, the acceleration rate slowed to almost zero. Since, in our sample, this period roughly coincides with ages 20–25 (
Figure 2), the end of a period of neuromaturational processes such as synaptic pruning and dendritic retraction (
40), the accelerated aging in schizophrenia patients may reflect accelerated neuromaturation. Interestingly, the individual variation in changes in brain age gap among patients was larger (SD=3.8 years) than in healthy subjects. This could be related to the same factors that play a role in healthy subjects as well, such as genetic background, cognitive functioning, and lifestyle, although the effect of some of these factors may be magnified in schizophrenia as a result of, for example, diminished cognitive challenges and reduced levels of physical activity. Nevertheless, our data also suggest that some of the accelerated aging of the brain in schizophrenia is related to the severity of the illness, since we found it to be positively associated with symptom and outcome scores and number and duration of hospitalizations. That progressive aging of the brain becomes less pronounced after the first years of illness could reflect the transition from a clinically unstable period, with large variability in functioning, to a relatively stable period, when patients have reached a plateau in functioning (
41,
42). The large variation in the pace of brain aging among the schizophrenia patients could thus be a reflection of this individual variation in the course of the disease and, related to that, lifestyle.
Applying our age prediction model in an independent sample, we found a zero brain age gap in healthy subjects and a significant gap (5.6 years) in schizophrenia patients. This replication at a different field strength (3 T) supports the generalization of our age prediction model. Interestingly, most brain areas that played a role in the prediction of age were not involved in the prediction of schizophrenia and vice versa. Many regions were found where lower gray matter density contributed to the greater age prediction, which is in line with the gray matter decreases found in aging brains (
43). These regions included the left and right caudate nucleus, the putamen, parts of the temporal lobes, the cerebellar vermis, and the left occipital pole. A few regions were found with the opposite effect: higher gray matter density contributed to greater age prediction in the posterior parts of the thalami. Whether these effects are true structural or physiological changes in gray matter or reflect degrading surrounding white matter tissue remains to be investigated. In the schizophrenia prediction weight map, widespread decreases in gray matter density were found at the interface between the left and right hemispheres and in the frontal lobe, the temporal lobe, the insula, and around the lateral ventricles. A number of marked positive associations between gray matter density and schizophrenia were also found in the left and right occipital poles, the putamen, and the globus pallidus. Interestingly, the latter two structures were the only regions that were enlarged in a meta-analysis of brain volumes in schizophrenia (
3).
The findings of this study should be considered in light of some limitations. Our brain age model is insensitive to any nonlinear changes of gray matter with age. Most patients had been ill for several years at the time of their first scan, so changes in brain age just after illness onset were based on a relatively low number of subjects. Patients with the poorest outcomes may not have been able to participate at follow-up (
22; but see also
25), which may have confounded the results on brain aging. Almost all patients used antipsychotic medication at the time of scanning, making it impossible to separate the effects of medication on aging of the brain from those of the illness. However, progressive brain changes in a sample of chronic, never-medicated schizophrenia patients (ages 20–70 years) have recently been reported (
44).
In conclusion, our data suggest that the widely reported progressive gray matter loss in schizophrenia reflects in part an accelerated aging of the brain that is quantitatively, but not qualitatively, different from that observed in healthy aging. While we also find brain abnormalities that are qualitatively different from those observed in healthy subjects, their evolution over time is highly variable. Thus, the progressive brain loss in schizophrenia appears to reflect two different processes: one relatively homogeneous, reflecting accelerated aging of the brain and related to outcome, the other more variable and specific, possibly reflecting individual variation related to the illness and to medication use. Differentiating between these two processes may not only elucidate the various factors influencing brain loss in schizophrenia, but also assist in individualizing treatment.