Poverty is one of the most powerful risk factors for poor developmental outcomes (
1–
7). Children who grow up in poverty have poorer cognitive and educational outcomes and are at a higher risk for a range of mental illnesses, including depression and antisocial behaviors (
8). The mechanisms by which poverty contributes to these negative developmental outcomes in children are multifaceted and include factors such as limitations on educational opportunities, family stress, and adverse environmental exposures, such as lead, cigarette smoke, poor nutrition, and air pollution (
3,
6,
9).
The clear evidence for the negative outcomes associated with poverty has led to a growing number of studies examining how the early experience of poverty influences brain development as a potential mediating pathway. This literature was summarized recently by Brito and Noble (
10), who identified evidence for a range of structural brain differences associated with various indicators of poverty (i.e., income-to-needs ratio, parent education), including reductions in whole brain gray and white matter volumes and reduced thickness in some brain areas. One of the most consistent findings has been an association between poverty indicators and reductions in hippocampal and amygdala volumes (
11–
19), with every study that examined hippocampal or amygdala gray matter volumes reporting significant effects. Notably, there is evidence that these alterations in hippocampal and amygdala volumes partially mediate the influence of poverty on later behavioral problems in children (
12).
These findings in humans are consistent with the animal literature showing effects of stress and environmental enrichment on hippocampal cell proliferation, dendritic length, and branching (
20–
23). The human and animal findings regarding the amygdala are more complicated. As noted above, poverty is consistently associated with reduced amygdala volume. However, animal studies show increased amygdala dendritic arborization following stress (
23,
24), and human studies of institutional rearing have also shown increased amygdala volume (
25). The differences in amygdala findings between poverty and institutional-rearing studies may reflect the fact that children living in poverty can have intact attachment relationships, and thus the mechanisms may differ. Nonetheless, it is possible that the hippocampus and amygdala volume reductions associated with poverty also influence the connectivity of these regions. Both the hippocampus and the amygdala show positive resting-state functional connectivity (i.e., correlated spontaneous fluctuations in blood-oxygen-level-dependent functional MRI [fMRI] measurements) with each other and other regions in the medial and anterior temporal lobes, as well with as the ventral medial prefrontal cortex (at age 10 or older for this region) (
26–
28). In addition, both the hippocampus and the amygdala show negative functional connectivity with left and right dorsal prefrontal and parietal regions (
28). These regions showing negative correlations with the hippocampus and amygdala are the same regions that are activated in studies of emotion regulation (
29,
30), and these anticorrelations are thought to indicate top-down regulation of emotion and stress responsivity supported by hippocampal and amygdala regions, suggesting a critical role for the integrity of these connections in mood and affective function.
Several studies have shown that early life stress and/or maternal deprivation are associated with altered connectivity between the amygdala and the ventral medial prefrontal cortex and pregenual anterior cingulate (
31–
33), as well as other regions (
34)—connections that are thought to be critical for effective emotion regulation. Furthermore, patterns of altered amygdala and/or hippocampal connectivity with the ventral medial and/or dorsolateral prefrontal cortex have also been seen in individuals with depressive psychopathology (
28,
35–
37). Given the large literature linking early stress to later depression (
38,
39) (although not selectively to depression), and given previous results of connectivity alterations associated with depression, these findings taken together could suggest that disruptions in amygdala or hippocampal connectivity may be one pathway by which early adversity contributes to risk for negative emotionality and depression. Few studies have investigated the effects of poverty on functional connectivity, although one showed that poverty predicted altered connectivity of the amygdala and medial prefrontal cortex while processing emotional faces (
40). However, to our knowledge, no studies have examined whether early poverty influences resting-state functional connectivity of either the amygdala or the hippocampus, which could reflect alterations in the integrity of intrinsic brain networks important for emotion responsivity and regulation.
The goal of the present study was to investigate the effects of poverty on childhood hippocampal and amygdala resting-state connectivity as a means to understanding whether alterations in these neural circuits may be one pathway contributing to the negative impact of poverty on child emotional outcomes and mental health. We also investigated whether poverty-related differences in amygdala or hippocampal connectivity predicted subsequent negative mood and depression severity and, if so, whether these differences in functional brain connectivity mediated the influence of poverty on subsequent negative mood.
Method
Participants
Participants were 105 children (54 of them girls) from a larger sample enrolled in the 12-year longitudinal Preschool Depression Study (N=305 at baseline, between ages 3 and 6). Children were invited to participate in the scanning portion of the study if they were psychiatrically healthy or if they had a history of clinical depression or anxiety (for additional detail, see the
data supplement that accompanies the online edition of this article). All study procedures were reviewed and approved by the Washington University School of Medicine Institutional Review Board. Children were included in the present study if they had usable functional connectivity data at the first imaging session (between ages 7 and 12), as described below, and had parent report data on income-to-needs ratio from the baseline Preschool Depression Study assessment. There were no significant differences in demographic variables (age, sex, income-to-needs ratio) between the imaging subsample and the original sample. There were no significant differences in sex, baseline income-to-needs-ratio, or negative mood/depression scores at the time of scanning between the children with and without usable functional connectivity data. However, the children with usable data were slightly but significantly older than those without (mean ages, 9.93 and 9.53 years, respectively; t=2.12, df=181, p=0.035).
Table 1 summarizes the demographic characteristics of the sample.
Measures
Income-to-needs ratio.
Income-to-needs ratio was operationalized as the total family income divided by the federal poverty level based on family size (
41). The value was calculated based on baseline Preschool Depression Study data on caregiver-reported total family income and total number of people living in the household.
Psychiatric diagnosis and symptom severity.
Participants were assessed annually using the Preschool Age Psychiatric Assessment (parent interview, ages 3–7) and the Child and Adolescent Psychiatric Assessment (parent interview, ages 8 and up; child interview, ages 9 and up) (
42; for further details, see reference
43). A negative mood/depression severity score at the time of study entry (preschool age, when income-to-needs ratio was measured) was computed by summing the number of core major depressive disorder symptoms (depressed mood, anhedonia, weight change, insomnia/hypersomnia, psychomotor agitation/retardation, fatigue, worthlessness/guilt, difficulty concentrating, suicidal ideation). A similar measure was created for school-age negative mood/depression severity, using items endorsed at the assessment wave closest to the scan. An anxiety severity score at the time of the scan was calculated using the core items from generalized anxiety disorder, separation anxiety, and posttraumatic stress disorder. An externalizing psychopathology score at the time of scanning was calculated using the core items from attention deficit hyperactivity disorder, oppositional defiant disorder and conduct disorder.
Neuroimaging
Participants completed a neuroimaging battery that included high-resolution structural, diffusion imaging, functional task, and resting-state scans collected on a Siemens 3-T TIM TRIO scanner at Washington University. The resting-state data from the first imaging session were the focus of the present analysis. T1-weighted structural images were acquired in the sagittal plane using a magnetization-prepared rapid gradient-echo (MP-RAGE) three-dimensional sequence (TR=2400 ms, TE=3.16 ms, flip angle=8°, 176 slices, field of view=256 mm, voxel size=1×1×1 mm). T2-weighted images were collected for registration purposes using a 3D-SPACE acquisition (TR=3200 ms, TE=497 ms, 160 slices, field of view=256 mm, voxel size=1×1×1 mm). Two resting-state fMRI scan runs were acquired in the vast majority of children (N=102 in the present sample; the other children ran out of time or were unable to stay still during the second run), each including 164 frames (∼6.8 minutes). Participants were instructed to rest with their eyes closed and to remain awake. Data were acquired using a spin-echo, echo-planar sequence sensitive to blood-oxygen-level-dependent (BOLD) contrast (T2*) (TR=2500 ms, TE=27 ms, field of view=256 mm, flip=90°, voxel size=4×4×4 mm, slices=36).
fMRI Preprocessing
Imaging data were preprocessed using the following steps: 1) correction for slice-dependent time shifts; 2) removal of the first four images of each run to allow BOLD signal to reach steady state; 3) elimination of odd/even slice intensity differences due to interpolated acquisition; 4) realignment of data acquired from each participant within and across scan runs to compensate for rigid body motion; 5) image intensity normalization to a whole-brain mode value of 1,000; 6) registration of the three-dimensional structural volume (T
1) to an atlas template (WU 711-2B) in the Talairach coordinate system using a 12-parameter affine transform and resampling to 1-mm cubic representation (
44,
45); 7) coregistration of the three-dimensional fMRI volume to the T
2, and the T
2 to the participant’s T
1 structural image; and 8) transformation of the fMRI data to 3×3×3 mm voxel atlas space using a single affine 12-parameter transform. Additional processing of the resting-state functional connectivity was done with in-house software and is described in more detail in the
online data supplement.
fMRI Analysis
We used FreeSurfer, version 5.1 (
46,
47), to create anatomical region-of-interest masks. The hippocampus and amygdala were segmented bilaterally from each participant’s T
1 anatomical image, downsampled to match the functional resolution of the atlas space (3×3×3 mm), and registered to the common atlas space. These images were summed, and a group-level anatomical mask was created by thresholding the region where at least half of the participants had overlap in their hippocampal and amygdala segmentations, allowing a more anatomically precise region of interest than relying on atlas regions of interest. The time-series from these four regions of interest were correlated with the time-series at every other voxel in the brain to create four whole brain voxel-wise correlation maps for each participant. Values in these maps were converted to z-statistics using Fisher’s r-to-z transform. These maps were used as the dependent measures described below.
Statistical Analysis
Normative connectivity patterns.
To establish the overall patterns of amygdala connectivity in our sample, two whole-brain one-sample t tests were run using in-house software (FIDL analysis package,
http://www.nil.wustl.edu/labs/fidl/index.html) (
48) to characterize significant voxel-wise resting-state functional connectivity (r-to-z transformed) with the left or right hippocampus and amygdala (patterns for the amygdala are reported in reference
49). Whole-brain t test results were thresholded based on Monte Carlo simulations (using 3dClustSim; afni.nimh.nih.gov/pub/dist/doc/program_help/3dClustSim.html) at z≥3 and ≥17 contiguous voxels to achieve a whole brain false positive rate of p<0.05.
To test our main hypotheses, we examined whole-brain regression analyses predicting voxel-wise functional connectivity with the left or right hippocampus and the left or right amygdala. After screening for outliers (none were above three standard deviations in univariate analysis, and none had Mahalanobis distance metrics greater than p<0.001 in multivariate analyses), baseline income-to-needs ratio was the predictor of interest, controlling for ethnicity (African American versus white and other versus white), sex (female versus male), and age at the time of scanning, with both income-to-needs ratio and age mean centered. Whole brain z-maps for the effects of income-to-needs ratio were thresholded as above based on Monte Carlo simulations at z≥3 and ≥17 contiguous voxels to control for multiple comparisons (whole brain p<0.05). Average connectivity values within each significant cluster were extracted for each participant to examine the relationship of these connectivity measures to potential mediators and outcomes.
To examine whether the regions showing relationships between income-to-needs ratio and amygdala/hippocampal connectivity related to negative mood/depression, we computed linear regressions using connectivity measures extracted from the identified regions of interest to predict negative mood/depression scores at the time of the scan, controlling for age and sex. To control false positives, we corrected for the number of regions identified within the amygdala connectivity analyses (p=0.05/7=0.007) and within the hippocampal connectivity analyses (p=0.05/4=0.0125). When these results were significant, we then tested whether either income-to-needs ratio or connectivity related to depression severity at the time of scanning even when accounting for depression severity at study entry (when income-to-needs ratio was measured). Significant effects in such analyses indicated a relationship to change in negative mood/depression severity over time. We then asked whether the connectivity measures mediated the relationship between income-to-needs ratio and negative mood/depression at the time of scanning, using the PROCESS procedure in SPSS (
50,
51), with age at the time of scanning, sex, and ethnicity as covariates.
Results
Characterizing Normative Hippocampal and Amygdala Connectivity Patterns
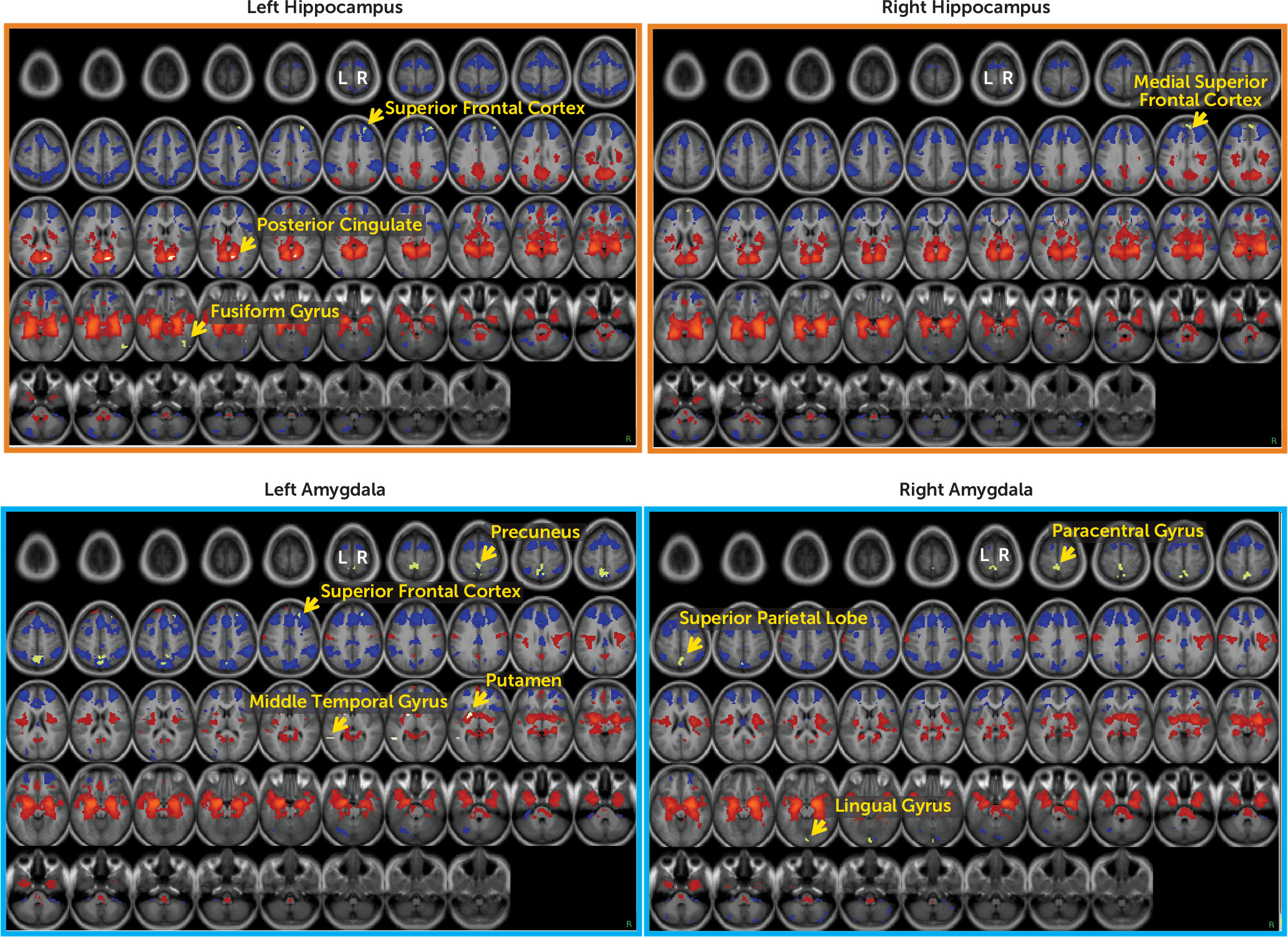
Figure 1 presents the results of whole-brain one-sample t tests of hippocampal and amygdala functional connectivity in this sample, with these seeds defined anatomically using FreeSurfer. Consistent with results reported in the literature, both the left and right hippocampus and amygdala showed strong positive connectivity with much of the subcortex, including contralateral homologous regions, striatum, brainstem, posterior insula, and ventromedial prefrontal cortex. Additionally, both the hippocampus and amygdala showed strong negative connectivity with much of the dorsomedial prefrontal cortex, lateral prefrontal cortex, anterior insula, cingulate cortex, and parietal lobe. The hippocampus also showed positive connectivity with posterior components of the default mode network (e.g., the posterior cingulate and the lateral parietal cortex).
Does Income-to-Needs Ratio Predict Hippocampal and/or Amygdala Connectivity?
Whole-brain z-maps for the effects of income-to-needs ratio, controlling for ethnicity, sex, and age at the time of scanning, are presented in
Table 2 and
Figure 1. Income-to-needs ratio negatively predicted connectivity between the left hippocampus and regions in the right fusiform and the right superior frontal gyrus, as well as between the right hippocampus and one region in the right superior frontal gyrus (higher income-to-needs ratio predicted more negative connectivity). In addition, income-to-needs ratio positively predicted connectivity between the left hippocampus and a region in the right posterior cingulate (higher income-to-needs ratio predicted more positive connectivity). Income-to-needs ratio also negatively predicted connectivity between the left amygdala and the right superior frontal gyrus and right precuneus as well as between the right amygdala and regions in the right lingual gyrus, left superior parietal lobe, and left paracentral gyrus. Income-to-needs ratio also positively predicted connectivity between the left amygdala and both the left putamen and the left middle temporal gyrus. For the majority of these connectivity patterns related to poverty, higher income-to-needs ratio predicted stronger connectivity in the normative direction (e.g., more positive for regions that typically show positive connectivity and more negative for those regions that typically show negative connectivity). The overlap between regions showing overall connectivity with either the hippocampus or the amygdala and those connections showing relationships to income-to-needs ratio are illustrated in green in
Figure 1. There were some regions showing functional connectivity relations to income-to-needs ratio that did not appear in the average functional connectivity maps, including left hippocampus to right fusiform, right hippocampus to medial superior frontal cortex, and left amygdala to middle temporal regions.
Does Poverty and/or Brain Connectivity Predict Negative Mood/Depression Severity at the Time of Scanning?
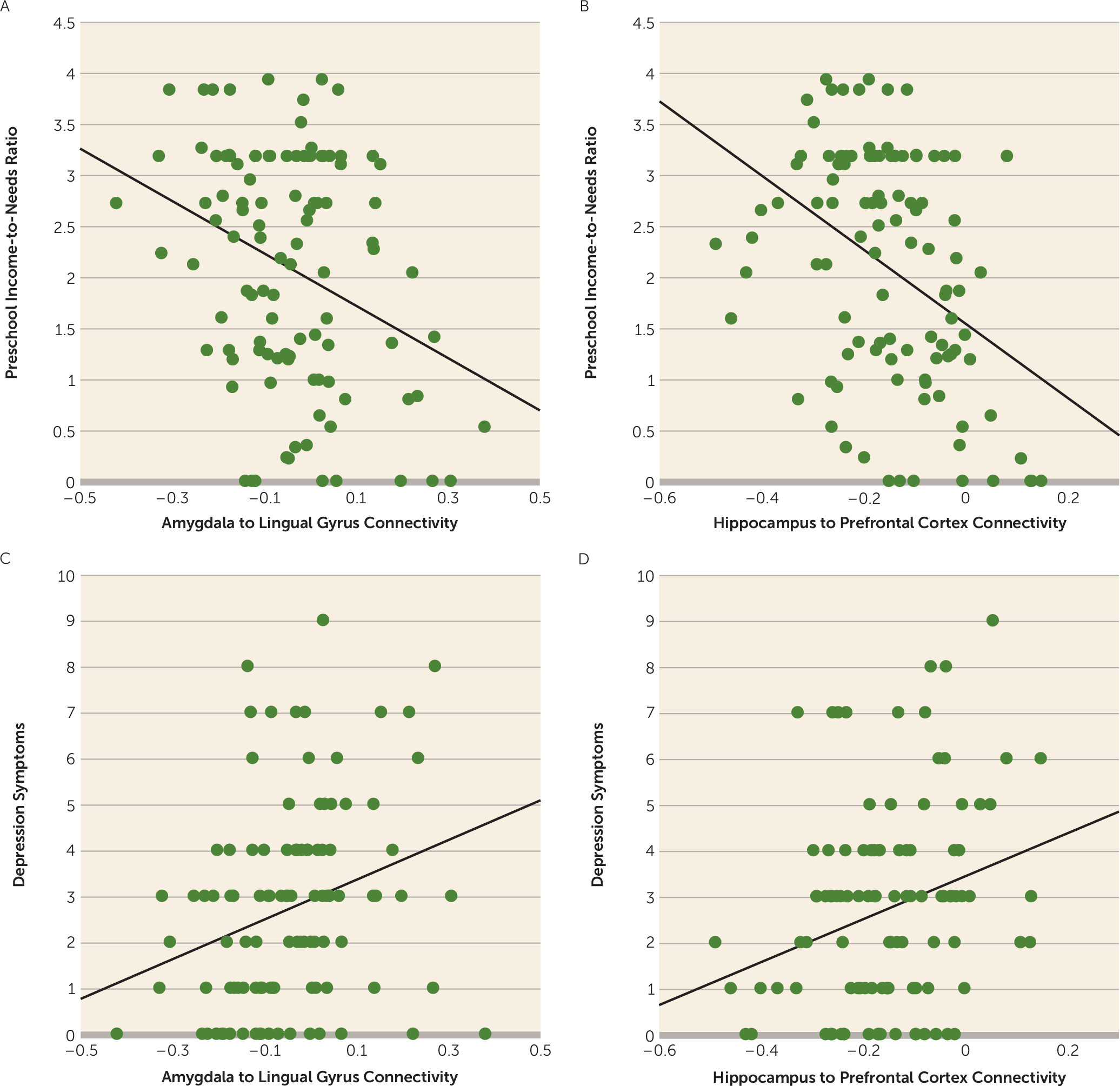
Income-to-needs ratio from study entry at preschool age significantly negatively predicted negative mood/depression severity at the time of scanning at school age (β=−0.27, p=0.03). In addition, connectivity between the right amygdala and the right lingual gyrus (β=0.28, p=0.004) and between the left hippocampus and the right superior frontal cortex (β=0.27, p=0.005) were associated with negative mood/depression after correcting for multiple comparisons. These relationships, illustrated in the scatterplots shown in
Figure 2, indicate that less negative connectivity (more positive) was associated with greater depression. Furthermore, both income-to-needs ratio and the connectivity measures continued to relate to negative mood/depression at the time of the scan even after accounting for negative mood/depression at preschool age (all p values, <0.05), indicating a relationship between preschool levels of poverty, connectivity, and a change (i.e., increase) in negative mood/depression over time.
Does Amygdala/Hippocampal Connectivity Mediate the Relationship Between Poverty and Negative Mood/Depression at the Time of Scanning?
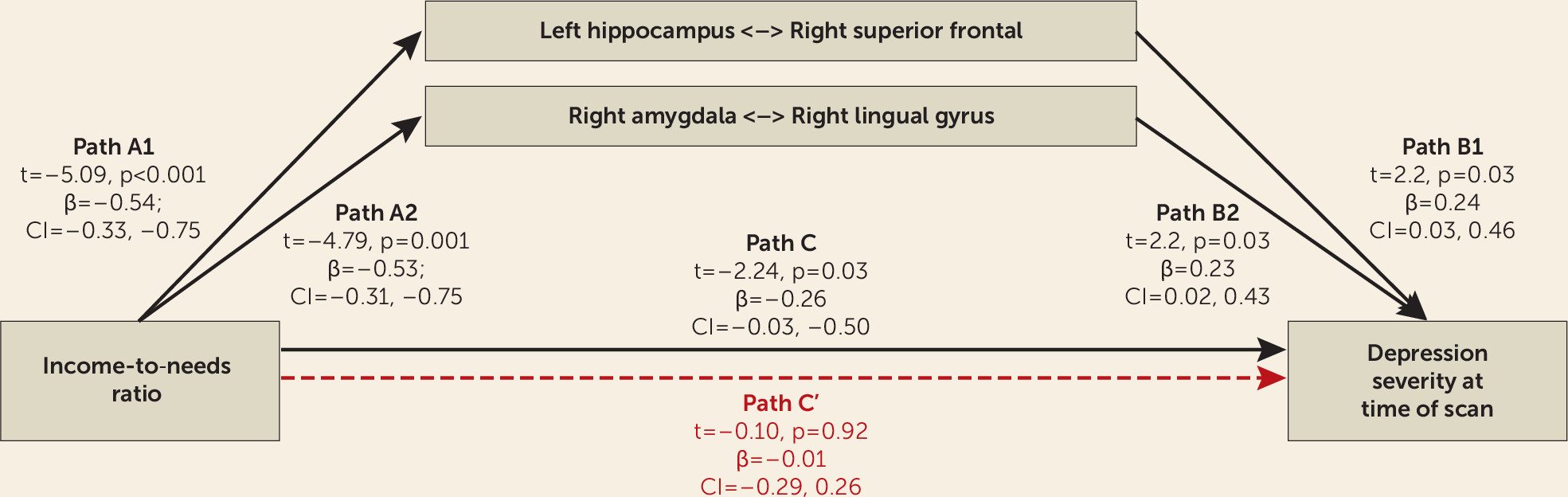
We next examined whether connectivity between the right amygdala and the right lingual gyrus and/or between the left hippocampus and the right superior frontal gyrus mediated the relationship between poverty and negative mood/depression measured at the time of the scan, since all three were related to depression in the analysis presented above. As shown in
Figure 3, hippocampal and amygdala connectivity significantly mediated the relationship between income-to-needs ratio and negative mood/depression at the time of the scan.
All of the relationships reported above hold if we control for whether or not the child’s mother had depression. In addition, as noted, numerous studies have found that poverty predicts hippocampal and amygdala volume, including our own previous work on the hippocampus. While poverty was related to reduced hippocampal and amygdala volume in the present sample (see the online data supplement), these volumetric changes do not account for the relationship between income-to-needs ratio and connectivity (see the data supplement). Furthermore, these relationships were not present for measures of anxious mood or externalizing psychopathology (see the data supplement).
Discussion
Our goal in this study was to investigate whether poverty was associated with alterations in the functional connectivity of either the amygdala or the hippocampus and, if so, whether these alterations were associated with severity of negative mood/depression. We found that income-to-needs ratio significantly predicted the connectivity of both the amygdala and the hippocampus bilaterally, a novel finding that has not previously been shown. For the majority of regions, lower income-to-needs ratio was associated with a reduction in the normative pattern of connectivity for these regions, such as reduced negative connectivity between the left hippocampus and amygdala and the right superior frontal cortex, functional connections thought to play a key role in emotion regulation. Furthermore, both income-to-needs ratio and amygdala/hippocampus connectivity were associated with the severity of negative mood/depression at the time of scanning, even after controlling for negative mood/depression at preschool age. These findings indicate that both poverty and connectivity were associated with an increase in negative mood/depression over time, as well as with overall severity levels at the time of scanning. Connectivity between the left hippocampus and the right superior frontal gyrus and between the right amygdala and the right lingual gyrus mediated the relationship between income-to-needs ratio and negative mood/depression at the time of scanning. Numerous studies have shown volumetric differences associated with poverty, including consistent reductions in amygdala and hippocampal volumes, and a recent large-scale study showed reductions in surface area associated with poverty (
52), which mediated links between poverty and cognitive function. Notably, the alterations in connectivity associated with income-to-needs ratio found in the present study were not secondary to an influence of poverty on amygdala or hippocampal volume, and they point to the importance of function as well as structure in the pathways by which poverty shapes child brain function and subsequent negative cognitive, emotional, and mental health outcomes.
Our results are consistent with a growing body of literature documenting that the early experience of poverty has a host of negative influences on child development and that at least some of these negative outcomes are mediated by the relationship of poverty to brain structure and function. The present findings identify a relationship between functional connectivity among regions known to be critical for effective emotion regulation as a mediator of the detrimental effects of poverty on negative emotional outcomes. In particular, one of our key findings was a relationship between lower income-to-needs ratio and reduced negative connectivity between the left hippocampus and amygdala and the right superior frontal gyrus. Patterns of anticorrelations between frontal regions and amygdala/hippocampal regions are thought to reflect top-down regulation of emotion and stress reactivity by cognitive control systems that can help implement effective emotion regulation (
29,
30). Of note, we do not mean to imply that these alterations in hippocampal-to-prefrontal connectivity associated with poverty reflect changes in structural connectivity, as regions can show functional connectivity even in the absence of direct structural connections. Furthermore, we do not suggest a unique relationship to either the left hippocampus or the superior prefrontal cortex, as examining images with a less conservative statistical threshold indicates that this area of connectivity extends down into the dorsolateral prefrontal cortex and is present for the right as well as the left hippocampal seeds, albeit at a less significant level. Nonetheless, the fact that the reduction in left hippocampus-to-right superior frontal connectivity was associated with negative mood/depression at the time of scanning is consistent with a hypothesized role for such connectivity patterns in emotion regulation and is consistent with other work showing a relationship between altered hippocampal-to-prefrontal connectivity and depression (
28). Furthermore, these findings are generally consistent with other published results from this sample, including reduced hippocampal and amygdala volumes associated with poverty (
11), reduced amygdala-to-cognitive control network connectivity among children who have a history of depression or who are at risk for depression (
35), and an interaction between genetic variation associated with increased stress reactivity and life events in predicting reduced hippocampal and amygdala volume (
53).
The finding that connectivity between the right amygdala and the right lingual gyrus also mediated the relationship between income-to-needs ratio and negative mood/depression at the time of scanning was somewhat more surprising. Our whole-brain analysis of the normative patterns of connectivity with the amygdala did not reveal significant amygdala-to-lingual gyrus connectivity, although average connectivity was significant, albeit modest, when examined as a region of interest (see
Table 2). Thus, it is not clear whether this relationship indicates the presence of anomalous positive connectivity only in individuals with low income and high depression, or the presence of negative connectivity only in those with high income and low depression. However, previous work on amygdala connectivity has demonstrated negative correlations between both lateral basal and superficial amygdala regions and the lingual gyrus, although in a somewhat more superior region than we saw (
26). The lingual gyrus has been related to visual form and word processing, but not typically with emotion processing. However, a growing number of studies have reported altered activity of the lingual gyrus in relationship to negative emotion processing in individuals with mood and psychotic disorders (
54–
57), particularly in relationship to the modulation of negative emotions. Combined with our current results, this previous work suggests the need for more focused research on the role of the lingual gyrus, and its connectivity with the amygdala, in emotion processing and emotion regulation.
A potential limitation of these data is that the original study sample was oversampled for preschoolers with symptoms of depression, which may limit generalizability. Specifically, we found that lower income-to-needs ratio was associated with higher negative mood/depression. Some previous work focusing on categorical diagnoses suggested that change in poverty had stronger effects on disruptive behavioral problems (e.g., conduct) than on emotional (e.g., depression/anxiety) problems in children (
58,
59). However, work in adults provides evidence for a causal relationship between poverty and depression (
60–
63). In general, rates of emotional disorders are much lower than rates of disruptive behavioral symptoms in children, potentially limiting the power to detect effects in childhood. Our sample was enriched for depression symptoms, which may have allowed greater power to detect such relationships with depression as compared with anxiety or disruptive disorders. Thus, it would be important in future work to replicate these results in a more epidemiological sample. It is also likely that the relationships between connectivity and depression are more complex and bidirectional, and the present study design cannot definitely address causality. Another limitation is that this analysis focused on income-to-needs ratio, and further work is needed to elucidate the impact of other factors that are also frequently associated with poverty. We did not find that our results were influenced by maternal support or life events (see the
online data supplement), but future work would benefit from including assessments of factors such as nutrition, school quality, and other indicators of enriched versus impoverished environments known to be associated with low income-to-needs ratio.
In summary, this study provides novel data suggesting that poverty influences functional brain connectivity in regions thought to be critical for emotion regulation, and that these changes in connectivity are a mediating factor by which poverty is associated with subsequent negative emotional and mental health outcome. These data add to the growing awareness of the immense public health crisis represented by the huge number of children growing up in poverty and the likely long-lasting impact this experience has on brain development and on negative mood and depression.