Cocaine is the second most commonly used illicit drug in Europe, particularly among young adult males, and it is often used in combination with alcohol and cannabis (
1). There are no registered pharmacological treatments for cocaine addiction (
2), although cognitive-behavioral therapy and contingency management have been shown to be fairly successful (
3). Learning processes, including Pavlovian and instrumental conditioning, play an essential role in the development and persistence of substance use disorder (
4). While these learning processes may form a potential treatment target (
5), psychotherapies targeting conditioning have not yet been shown to be effective (
6). A possible reason is that previous research has focused primarily on the learning mechanisms underlying appetitive conditioning (cue exposure treatment), while aversive conditioning may be equally important in the etiology and treatment of cocaine use disorder.
Through the process of appetitive conditioning, drug responses become associated with drug-related cues. When these drug-related cues are subsequently encountered in an abstinent state, they can trigger the retrieval of memories of previous drug experiences and thereby induce cue reactivity, craving, and drug-seeking and drug-taking behavior (
7,
8). However, there is increasing evidence that aversive conditioning plays an equally important role in substance use disorder (
9). First, stress-induced relief craving is a frequently observed phenomenon in addiction (
10,
11), and addicted individuals are thought to take drugs to avoid aversive states such as stress (
12,
13). Through the process of aversive conditioning, external stimuli can become associated with internal stress states, thereby (indirectly) motivating drug-seeking and drug-taking behavior itself (
5,
14). Second, there is a high comorbidity between anxiety disorders and substance use disorder (
15). Since abnormalities in aversive conditioning and extinction learning have been reported in anxiety disorders (
16), similar abnormalities are expected to be found in substance use disorder. And third, the neural underpinnings of aversive and appetitive conditioning are thought to largely overlap, since the mesolimbic dopamine system is a key player in both types of conditioning and extinction learning (
9). As a consequence, neuroadaptive changes within the mesolimbic dopamine system due to stress or drug use may also modulate the processing of appetitive conditioning or aversive conditioning, respectively (
17–
19). Knowledge about the role of aversive conditioning in substance use disorder could thus have important implications for understanding its pathophysiology and the development of prevention and treatment strategies. However, so far no studies have been reported on the neural and physiological underpinnings of aversive conditioning in substance use disorder.
From studies in healthy individuals, we know that aversively conditioned stimuli evoke an increase in skin conductance response (
20) and activation of the neural fear network, including the amygdala, dorsomedial prefrontal cortex, and insula, and deactivation of the ventromedial prefrontal cortex (
21,
22). Within this network, the amygdala, dorsomedial prefrontal cortex, and insula are involved in the expression of aversive conditioned responses, while the ventromedial prefrontal cortex is involved in the inhibition of conditioned behavior (
21,
23,
24). Anxiety disorders are characterized by hyperresponsiveness of the amygdala, dorsomedial prefrontal cortex, and insula during fear learning and hyporesponsiveness of the ventromedial prefrontal cortex during extinction learning, reflecting the presence of enhanced fear learning and impaired fear extinction capabilities (
25). Counterintuitively, these differences in neural plasticity are typically not associated with enhanced differential skin conductance responses (
16,
26,
27).
In this study, we investigated the physiological (skin conductance responses) and neural (regional brain activation) correlates of fear conditioning and extinction in cocaine abusers and non-drug-using control subjects. While the neural and physiological underpinnings of aversive conditioning and extinction learning have not yet been investigated in substance use disorder, previous studies have demonstrated that substance use disorder is associated with hyperresponsiveness of the dorsomedial prefrontal cortex, insula, and amygdala to conditioned drug cues, a response that is slowly extinguished (
6). We therefore hypothesized that cocaine abuse is associated with enhanced fear conditioning and impaired extinction learning, as reflected by hyperactivation of the amygdala, insula, and dorsomedial prefrontal cortex during fear conditioning and hypoactivation of the ventromedial prefrontal cortex during extinction learning as compared with control subjects.
Discussion
Although aversive conditioning and extinction learning have been suggested to play an important role in the development and persistence of drug abuse (
5,
14,
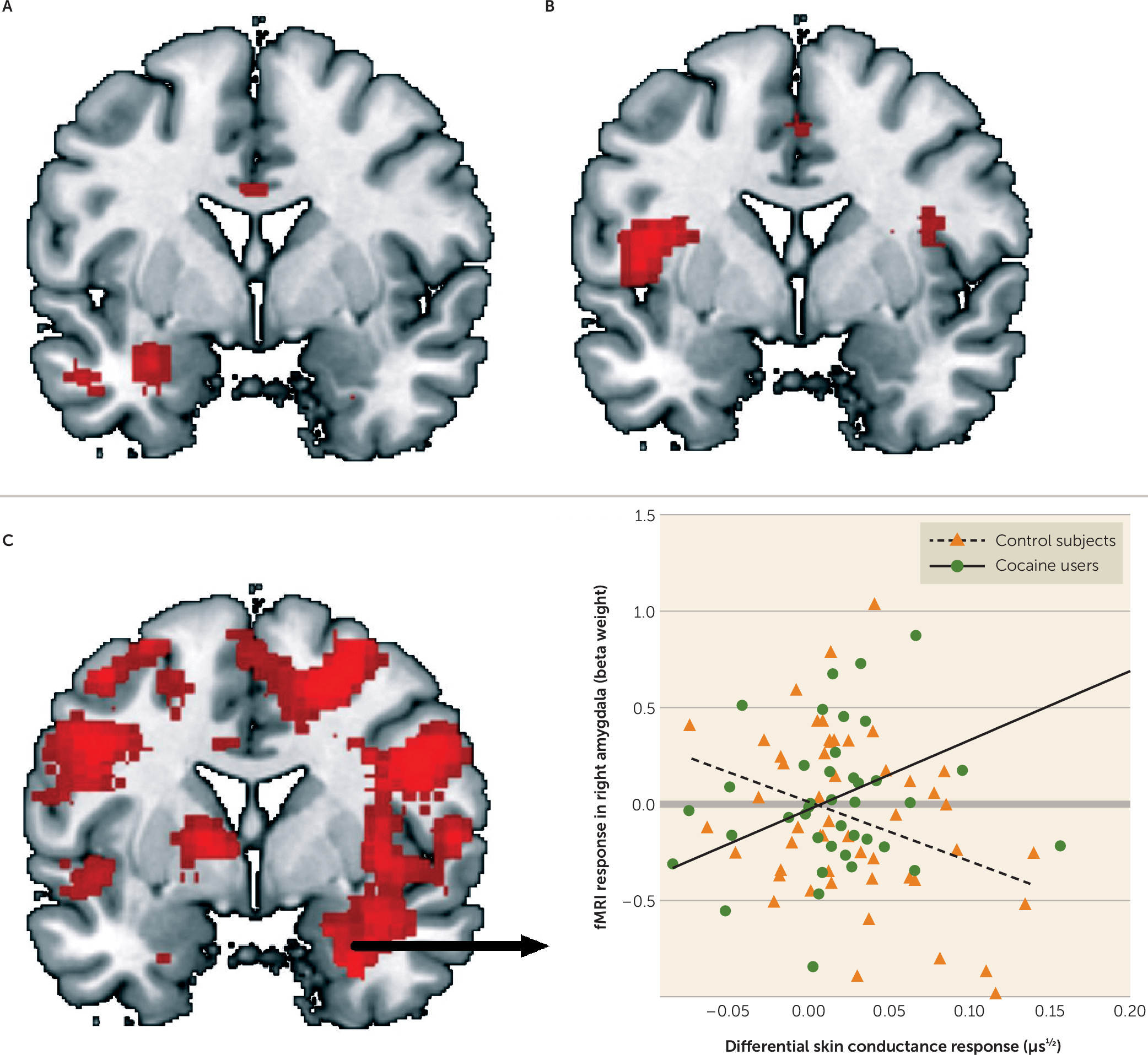
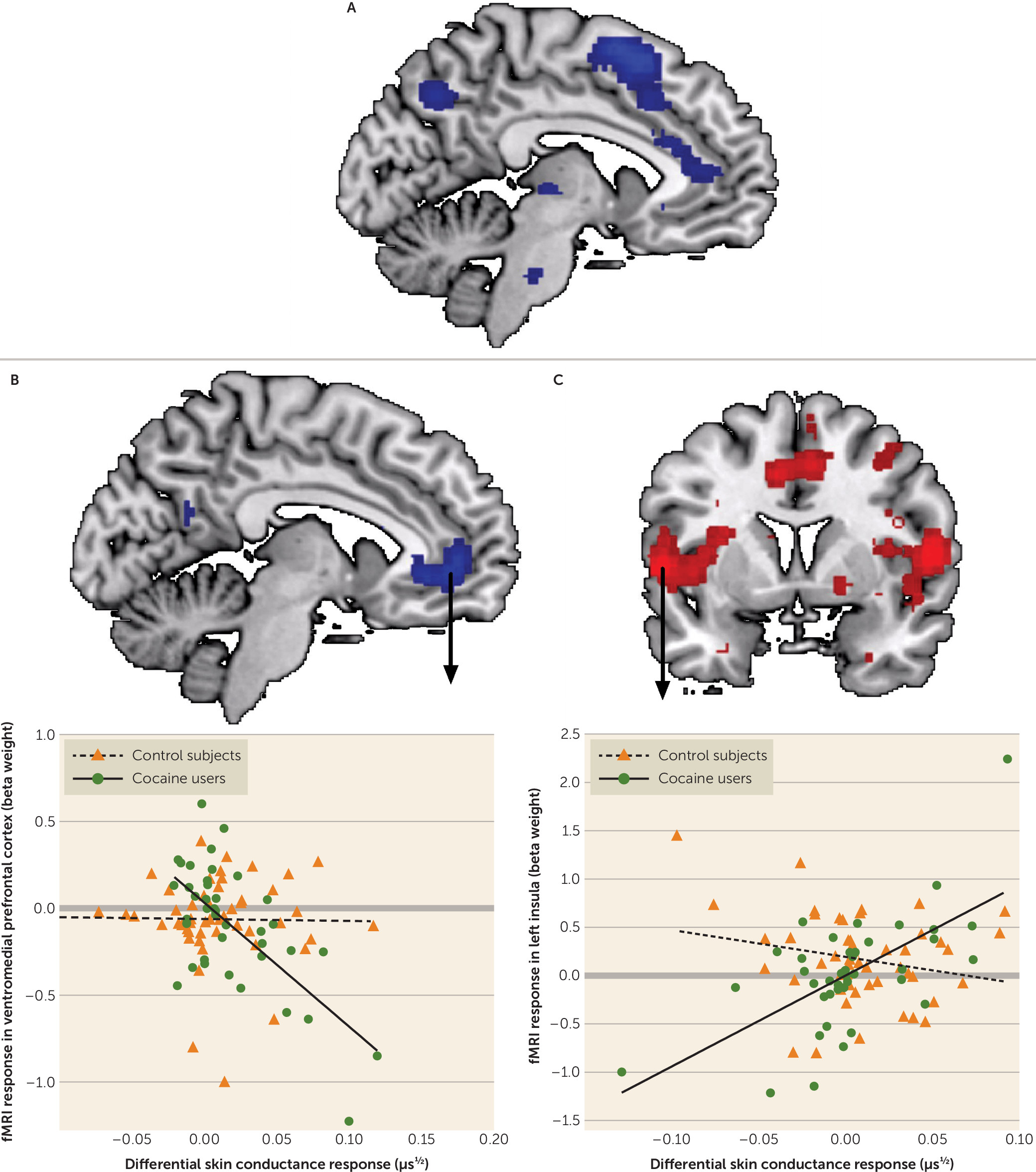
24), this is, to our knowledge, the first study addressing the physiological and neural mechanisms underlying aversive conditioning and extinction learning in substance use disorder. Consistent with the hypothesis of enhanced aversive conditioning in substance use disorder, cocaine users showed hyperactivity of the amygdala and insula during aversive conditioning compared with control subjects. Inconsistent with the hypothesis of impaired extinction learning, cocaine users showed hypoactivation of the dorsomedial prefrontal cortex during late extinction, suggesting enhanced extinction learning compared with control subjects.
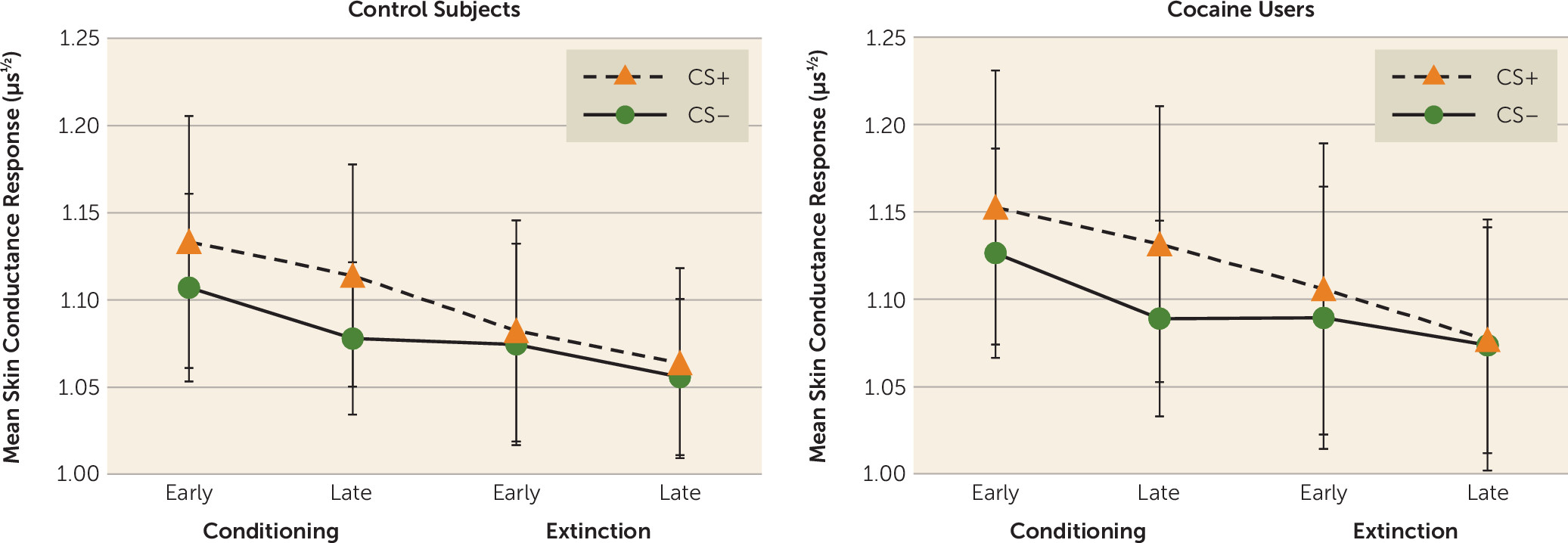
While there were no group differences in skin conductance response during aversive conditioning or extinction learning, skin conductance response and activation of the neural fear network were more strongly correlated across subjects in cocaine users than in control subjects, suggesting that the emotional response to conditioned stimuli is stronger in cocaine users than in control subjects. Overall, these findings support the postulated role of abnormal aversive learning processes in substance use disorder.
The amygdala is important for the rapid encoding of new stimulus-threat relationships (
22), while the insula modulates the visceral response to conditioned stimuli (
24). The dorsomedial prefrontal cortex is suggested to be involved in conscious negative appraisal of threat. These brain regions are key structures within a neural fear network, and previous studies have repeatedly demonstrated that activation of these regions is associated with fear conditioning and extinction learning (
22), is enhanced in individuals with enhanced fear learning and impaired extinction learning (
25), and is related to other measures of conditioned behavior, including skin conductance response (
20). The finding of increased amygdala and insula activation during fear conditioning and reduced dorsomedial prefrontal cortex activation during late extinction in cocaine users therefore suggests that cocaine users exhibit enhanced fear conditioning as well as enhanced extinction learning compared with control subjects.
Increased amygdala activity in cocaine users during early conditioning may reflect increased attention for threat-related stimuli and enhanced aversive conditioning, followed by increased activity of the insula during late conditioning, which may reflect enhanced visceral processing. Increased responsiveness of these structures within the neural fear network are thought to underlie negative reinforcement mechanisms in substance use disorder, stress-induced relief craving, and subsequent continuation of or relapse into drug use (
13,
18,
24,
33). Abnormalities in the neural underpinnings of aversive conditioning in cocaine users may therefore reflect a risk for cocaine abuse and relapse.
In contrast to our hypotheses, cocaine users showed reduced activity of the dorsomedial prefrontal cortex during late extinction, suggesting the presence of superior, and not impaired, extinction learning. While enhanced fear extinction learning may be beneficial in anxiety disorders, it may actually form a risk for the persistence of drug use, as it may underlie an inability to modify behavior in response to negative outcomes, resulting in risky behavior (
34). Future studies should, however, investigate whether these difference are also present after reinstatement of the fear conditioned response. Irrespective of whether enhanced extinction of neural fear conditioned responses is good or bad, our data demonstrate that the neural underpinnings of fear extinction learning in cocaine users differ from those in patients with an anxiety disorder (
16,
21,
22,
27,
35). While the relation between enhanced fear extinction learning and stress relief craving remains to be investigated, these findings may explain why cue exposure treatment, which is a successful treatment strategy in anxiety disorders, is not as effective in addiction (
6).
In line with the typical observation in anxiety disorders, we found no group differences in differential skin conductance responses (
16,
26,
27). Although these results could be interpreted as less efficient neural processing in cocaine users, there is substantial evidence that hyperactivation of the amygdala, insula, and dorsomedial prefrontal cortex reflects the persistence of an increased expression of conditioned fear (
22). However, there are several other explanations for the dissociation between the skin conductance response and fMRI results. First, differential skin conductance responses are suggested to be more dependent on higher cognitive levels of learning, whereas differential activation of the neural fear network is suggested to be independent of higher cognitive processing (
20). This would suggest that cocaine abuse is associated with abnormalities in fear conditioning and fear extinction that are mainly dependent on unconscious processes. Furthermore, the finding that the skin conductance response and fMRI data are significantly correlated in cocaine users but not in control subjects may indicate that skin conductance responses in cocaine users are less dependent on conscious processing of conditioned cues. Alternatively, it has demonstrated that the amygdala plays a critical role in the modulation of skin conductance responses to threat (
36). Therefore, these results may indicate that cocaine users have a stronger emotional response to stimuli that predict an aversive outcome. In addition, several studies in anxiety disorders found that although there were no group differences in skin conductance responses during fear conditioning or extinction learning, differences were present during extinction recall (
27). Thus, it could be that differences in neural processing precede differences in skin conductance responses, which can be detected only during extinction recall. Altogether, skin conductance responses may not be sensitive and fear-specific enough to detect small group differences during fear conditioning or extinction learning (
37).
While sensitivity to stress has long been known to be an important risk factor for substance use and relapse (
12), this is one of the first studies to investigate the potential neural mechanisms that underlie this phenomenon. We demonstrated that the neural fear network of regular cocaine users is hyperresponsive to cues that predict a negative outcome. These findings emphasize that in addition to reducing drug-conditioned responses (reward craving), treatment should also try to reduce the (neural) sensitivity to stressors (relief craving). This could be achieved by means of cognitive-behavioral treatment (e.g., mindfulness-based relapse prevention [
38]) or pharmaceutical treatments that target the noradrenergic stress system (e.g., propranolol [
39]).
An important strength of this study is the large sample size and the assessment of the potential confounding effect of state anxiety. However, the study also has limitations. First, because only male participants were included in the study, these results may not generalize to female cocaine users. Second, because of the cross-sectional design, we need to be cautious with statements about the causality of our findings, and future studies should examine whether increased neural sensitivity for aversive events is a risk factor for cocaine abuse, a consequence of cocaine abuse, or a combination of the two. Third, most cocaine users in our sample also used cannabis, alcohol, and nicotine on a regular basis, thereby making it impossible to tell whether differences in aversive conditioning are related to cocaine, cannabis, or alcohol use or to some combination of these. Nevertheless, the exploratory analysis suggested that hyperresponsiveness of the amygdala and insula during aversive conditioning and hyporesponsiveness of the dorsomedial prefrontal cortex during late extinction learning are independent of the type or amount of substance used. Moreover, as polysubstance use is common among cocaine users in treatment (
1), we expect that our sample reflects typical cocaine users. Fourth, we tested for group differences during early and late conditioning, as well as early and late extinction, while there was no phase-by-group interaction effect. Although this is in accordance with most studies in the field (
22), it should be noted that such statistical flexibility could increase type I errors. Finally, while the neural pathways that underlie aversive and appetitive conditioning and extinction overlap, more research is needed to investigate whether and how enhanced neural sensitivity to aversive conditioned cues and enhanced sensitivity to drug-conditioned cues are related.
In summary, we found that cocaine use disorder is associated with hyperresponsiveness of the neural fear network during fear conditioning and extinction learning, possibly reflecting enhanced fear learning. Although the relationship between aversive conditioning and stress-induced relief craving remains to be investigated, this study is an important contribution to the understanding of the role of aversive conditioning in, and the etiology of, substance use disorder.