Bipolar II disorder is recognized as a distinct subtype from bipolar I disorder (
1). Most patients with bipolar II disorder spend substantially more time in the depressed than in the hypomanic phase of their illness (
2,
3). Some patients experience not only more depression but up to triple the time depressed relative to hypomanic (
3). The management of patients with bipolar II disorder can be complex when they present with depression. The initial treatment recommendation might include a mood stabilizer alone or in combination with an antidepressant. Some patients, however, may feel that their hypomanic symptoms do not require treatment (
4). They may thus be averse to taking a mood stabilizer and may inquire about being treated solely with an antidepressant. Antidepressant monotherapy is not recommended for patients with bipolar I depression because of high rates of switch to mania (
5–
8). Treatment recommendations vary for bipolar II depression (
9–
16), but concerns remain that antidepressant use as monotherapy or in combination with a mood stabilizer could increase the risk for switch to hypomania or cause cycle acceleration.
Clinicians, however, routinely use antidepressants as part of their treatment regimen for bipolar disorder (
7,
17). Three studies suggest lower antidepressant-induced switch rates in persons with bipolar II depression compared with bipolar I depression when treated with an antidepressant
in combination with a mood stabilizer (
18–
20). It has not been well studied whether monotherapy with a selective serotonin reuptake inhibitor (SSRI) will destabilize bipolar II patients with depression more than mood stabilizer monotherapy or combination therapy with a mood stabilizer (
21).
While there are now three pharmacological treatments approved by the U.S. Food and Drug Administration (FDA) for bipolar I depression (olanzapine-fluoxetine, quetiapine, lurasidone), there is only one FDA-approved treatment for bipolar II depression (quetiapine). Given the high rates of functional impairment (
22,
23) and the risk for suicide (
24–
28) in bipolar II patients with depression, more studies are needed to find treatment options that optimally help patients without causing harm.
To address the question of treatment options for bipolar II disorder, we conducted a 16-week randomized double-blind trial to determine whether sertraline monotherapy would be associated with higher switch rates than lithium monotherapy or lithium/sertraline combination therapy. We secondarily sought to determine whether treatment response or treatment-emergent side effects differed among lithium monotherapy, sertraline monotherapy, or combination therapy. We also hypothesized that the combination therapy would accelerate treatment response relative to the monotherapies.
Method
This randomized double-blind study was conducted from July 2006 to June 2013 at four clinical sites: UCLA Medical Center and VA Greater Los Angeles Healthcare System; University of Cincinnati College of Medicine and the Lindner Center of HOPE; University of Texas Southwestern Medical Center; and the Veterans Affairs Palo Alto Health Care System. The protocol was approved by the local institutional review board at each site, and a data safety monitoring board oversaw the conduct of the trial. Participants provided written informed consent after receiving a complete description of the study.
Participants
Potential participants were identified from outpatient treatment facilities at each site and the surrounding communities through advertisements. Participants had to be between 18 and 65 years old and meet DSM-IV criteria for bipolar II disorder, current major depressive episode, as determined by the Structured Clinical Interview for DSM-IV (SCID) (
29) (interrater kappa values were ≥0.80). Additional entry requirements included a score ≥22 (moderate or greater severity) on the Inventory of Depressive Symptomatology–Clinician Rated (IDS-C) (
30), a score ≥3 on the depression severity subscale of the Clinical Global Impressions Scale for Bipolar Disorder (CGI-BP) (
31), a score ≤8 on the Young Mania Rating Scale (YMRS) (
32), and a mania severity subscale score of 1 (not ill) on the CGI-BP (intraclass correlation coefficients were >0.7 on the IDS-C, YMRS, and CGI-BP).
Exclusion criteria included current mixed symptoms or psychosis, suicidality (a score ≥2 on IDS-C item 18), a substance use disorder within the previous 3 months, and a past history of nonresponse to lithium or sertraline for depression with ≥6 weeks of treatment at an adequate dosage. The presence of rapid cycling and psychosis was determined by SCID interview.
Treatment Procedures
Participants were assessed weekly for 6 weeks and then every 2 weeks for 10 weeks to monitor dosing and side effects and to administer the IDS-C, the YMRS, the CGI-BP, and a side effect checklist. Minimum target dosages were 100 mg/day for sertraline and 900 mg/day for lithium, with dosage changes dictated by clinical response and lithium serum levels, obtained at least three times during the study period, with the first assessment 2 weeks after medication initiation. We attempted to keep serum levels between 0.8 and 1.2 mEq/L, based on guidelines for bipolar I disorder. However, because precise therapeutic serum levels for patients with bipolar II depression are unknown, we allowed participants to remain in the study if they could only tolerate serum levels lower than 0.8 mEq/L. On the side effect checklist, participants rated 40 potential side effects as absent, mild, moderate, or severe.
Sample Size, Randomization, and Blinding Procedures
A total of 142 participants were block-randomized in a 1:1:1 ratio in blocks of nine, stratified by site and rapid cycling status. The random number sequences were generated by the Semel Institute Biostatistics Core using a customized program and provided in the form of an Excel spreadsheet to the unblinded UCLA project manager, who in turn distributed the sequences to the UCLA and University of Cincinnati pharmacists and to the University of Texas Southwestern Medical Center and Veteran Affairs Palo Alto Health Care System unblinded project manager. Participants and all personnel who rated or interviewed them were blind to medication assignment. Each participant received two blinded pills per day: either one placebo and one active pill or two active pills. At each site, one unblinded nonrating physician was designated to review lithium levels and categorize the level as “subtherapeutic (≤0.6 mEq/L),” “therapeutic (>0.6 to 1.2 mEq/L),” or “toxic (>1.2 mEq/L).” That physician provided this information to the blinded study physician, who used it in combination with mood ratings and clinical interview to determine whether a dosage change was needed. To maintain the blind, serum levels were obtained from all participants. For participants receiving sertraline monotherapy, the unblinded physician indicated a category appropriate to the prescribed dosage and clinical symptoms (subtherapeutic, therapeutic, toxic). (For a participant flow diagram, see Figure S1 in the data supplement that accompanies the online edition of this article.)
Safety and Early Study Termination
Criteria requiring early study termination included a switch to mania; presence of suicidal ideation (a score >2 on IDS-C item 18 or a score of 2 with a plan, or as assessed by the SCID mood module and/or clinical interview); hospitalization; medication intolerance; an adverse event requiring a change in the medication regimen; and a relapse of substance abuse or dependence. Participants experiencing a hypomanic switch (operationalized below) were allowed to remain in the study if their symptoms were not considered by the clinician, the participant, or the participant’s family members to be causing significant distress.
Primary and Secondary Outcomes
Primary outcome: switch to hypomania or mania.
At any study visit, a switch to hypomania was defined as a YMRS score ≥12 and a CGI-BP mania severity score of 2 (minimum) or 3 (mild). A switch to severe hypomania was operationalized as a YMRS score ≥14 and a CGI-BP mania severity score ≥4 (“moderately ill”). Switch to mania was defined as developing a manic episode as defined by DSM-IV and confirmed by SCID interview.
Secondary outcome: treatment response.
Treatment response was operationalized as either a decrease of ≥50% in IDS-C score or a decrease of ≥2 points in CGI-BP depression severity score, relative to baseline, for at least two consecutive visits spanning at least a 2-week period. All treatment responses were confirmed as not due to hypomanic switch. Rates of and time to treatment response were compared across the course of the study, with time indexed by the number of days from treatment initiation to the beginning of the first interval for which response criteria were met. We also recorded the change in manic and depressive symptom scores from baseline to the study exit visit. Weekly IDS-C and YMRS scores were analyzed as secondary outcomes to provide a more detailed picture of the trajectory of treatment-related improvements.
Secondary outcome: treatment-emergent side effects, tolerability, and dropout.
Side effects on the 40-item checklist were aggregated into six categories: thirst, tremor, gastrointestinal, urinary frequency, sexual functioning, and CNS effects. Side effects were designated as treatment emergent if they began after randomization or became more severe than baseline values and were moderate or severe in intensity. Rates of treatment-emergent side effects, both overall and in each category, were compared, adjusting for time in study. Reasons for dropout were categorized as treatment-emergent side effects as well as mood related, lost to follow-up, and other (see Table S2 in the online data supplement). Mood variability (subthreshold hypomanic and depressive symptom cycles) during the treatment period is a secondary outcome that will be explored in a future report.
Statistical Analysis
Chi-square tests and analyses of variance (or nonparametric equivalents as needed) were used to compare the treatment groups on baseline demographic and clinical characteristics. Measures that showed significant differences and were associated with the primary outcomes were included as covariates in subsequent analyses. Preliminary analyses were conducted including site main effects and site-by-treatment interactions to check for differential implementation of the intervention or evaluation of the outcomes. To increase power and precision of estimates for the other parameters, site terms were dropped from subsequent models if they were not significant in preliminary analyses.
We checked for differential patterns of dropout by treatment group, site, and baseline demographic and clinical characteristics using backward stepwise proportional hazards regression. Significant predictors of dropout were included in subsequent models to minimize the potential for bias. In addition, since the ability to complete the study protocol while remaining on the assigned medication was itself a measure of tolerability, we compared time to and rates of overall dropout across the treatment arms and assessed whether intolerability of side effects was the primary reason for dropouts. All tests were two-tailed at an unadjusted alpha of 0.05. Since our primary goal was not to establish superiority of one treatment to another but rather to determine whether sertraline monotherapy could be associated with higher switch rates than lithium-based treatments, it was more conservative to minimize type II as opposed to type I errors.
Our primary analyses consisted of 1) survival models comparing rates of switching (primary outcome), 2) treatment response across the treatment groups over time, accounting for censoring, and 3) logistic regression models for development of medication-induced side effects. Specifically, nonparametric (Kaplan-Meier) estimates of the raw survival functions were obtained for each study arm, and log-rank tests were used to check for differential treatment effects. Cox proportional hazards regression models were then used for survival analyses with covariate adjustment. Since rapid cycling has been associated with poorer outcome (
33), we stratified the randomization based on rapid cycling status and examined rapid cycling status interactions with treatment group. To characterize the trajectories of treatment-related improvements on symptom measures collected weekly (YMRS, IDS-C) we used generalized linear mixed models with medication group, time, and a group-by-time interaction. We used random intercepts to account for the participant-level effects and, since antidepressants take approximately 6 weeks to reach full therapeutic effectiveness, allowed for a change in slope at this point.
Treatment-emergent side effects (present or absent) were recorded as aggregate measures over the course of the study and were compared across treatment arms using standard logistic regression, adjusting for time in study and the covariates indicated above. Finally, two exploratory analyses were performed. First, we compared sertraline peak dosage and lithium serum levels by treatment group (lithium or sertraline monotherapy, respectively, versus combination therapy), treatment response, and switching status, adjusting for rapid cycling status and baseline covariates. For sertraline, we used logistic regression to examine whether or not participants reached the minimum target dosage of 100 mg/day. For lithium, since participants had different numbers and spacing of serum level readings, generalized linear mixed models were fitted with random intercepts and adjusted for time of measurement, rapid cycling status, and baseline covariates. To allow for titration to the optimal medication dosage, only measurements taken more than 2 weeks after treatment initiation were included. Second, we considered the effects of substance abuse or dependence on our major outcomes using the survival models and adding indicators for any history of abuse or dependence of alcohol or drugs. Where we found significant drug effects, we also explored breaking them out by category, including cannabis and stimulants (e.g., amphetamines, cocaine).
Results
Baseline Characteristics
The participants’ demographic and clinical characteristics are summarized by group in
Table 1, and by site in Table S1 in the
online data supplement. Of the 142 participants, six (4.2%) had psychotic symptoms during a past depressive episode and 59 (41.6%) were current rapid cyclers. There were significant between-site differences in race, ethnicity, and baseline YMRS and CGI-BP depression severity scores. However, there were no significant differences between treatment groups at baseline except on the IDS-C (F=3.07, df=2, 139, p=0.049), which was included as a covariate in subsequent models. Preliminary analyses showed a main effect of site on switching (Cox regression χ
2=6.72, df=2, p=0.035), so site was retained as a predictor in subsequent models for this outcome. There were no group-by-site interactions for treatment response, symptom measures, treatment-emergent side effects, or premature study discontinuation, so these terms were omitted from subsequent models.
Premature Study Discontinuation
Descriptive statistics for early discontinuations overall and those due to treatment-emergent side effects are presented in Table S2 in the
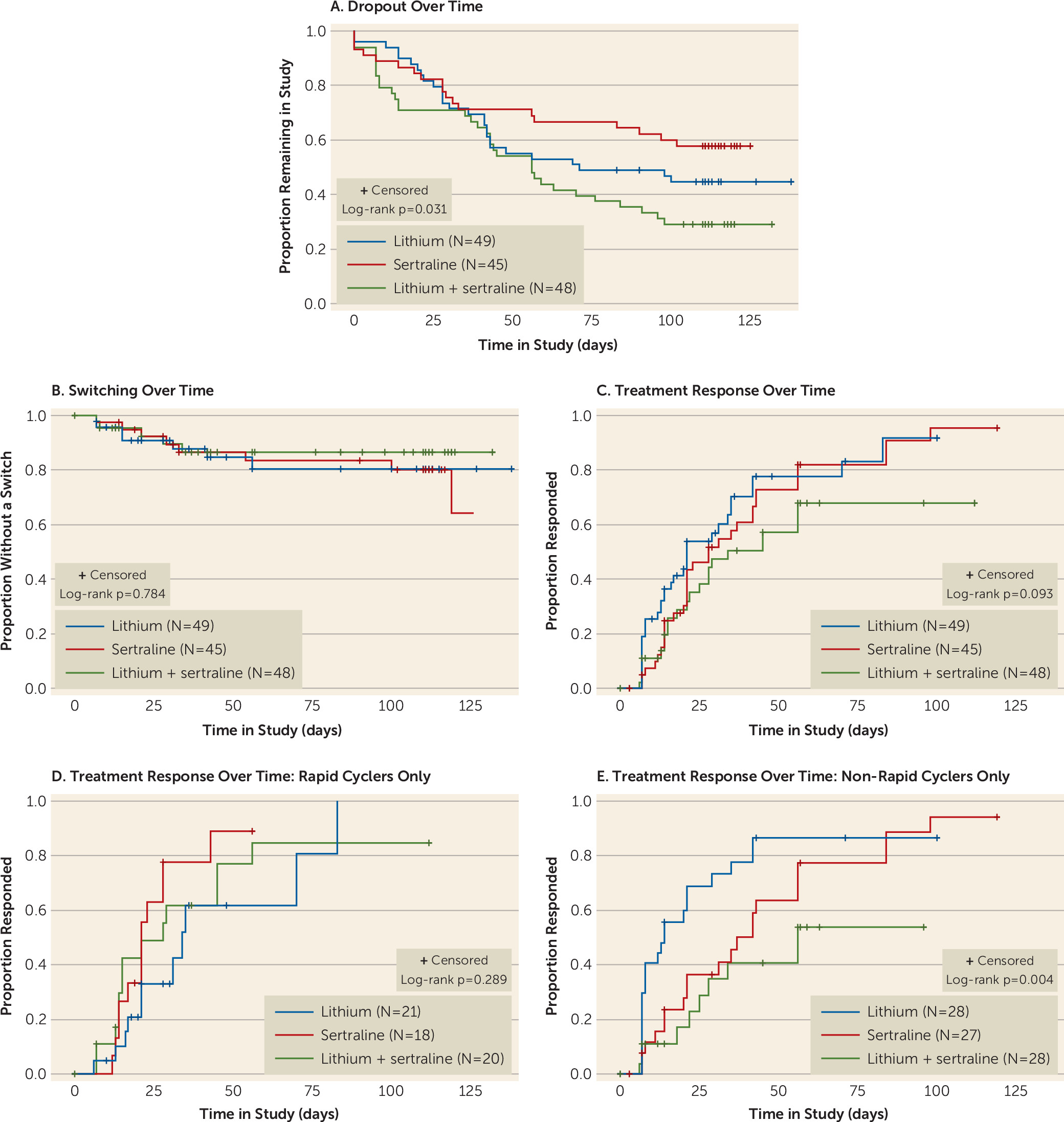
data supplement. There were no significant differences between groups in the likelihood of developing a treatment-emergent side effect, but the overall dropout rate was significantly higher in the lithium/sertraline combination arm, the treatment most commonly recommended in clinical practice. The corresponding Kaplan-Meier estimates of the survival curves are depicted in
Figure 1A. The sertraline monotherapy group had the lowest rate of early discontinuation (42.2%), followed by the lithium monotherapy group (55.1%) and then the combination therapy group (70.8%) (log-rank test χ
2=6.94, df=2, p=0.03). In backward stepwise proportional hazards regression analyses, the only predictors of study discontinuation that were significant or approached significance were treatment group (χ
2=5.93, df=2, p=0.052), younger age at onset of depression (χ
2=3.16, p=0.075), and lower baseline CGI-BP depression severity score (χ
2=5.79, df=1, p=0.016). As a result, baseline CGI-BP depression severity score and age at onset of depression were included as covariates in the subsequent models for the primary treatment outcomes.
Switching
Descriptive statistics for switching are presented in
Table 2, and the corresponding Kaplan-Meier estimates of the survival curves in
Figure 1B. Of the 142 participants, 20 (14%) experienced a switch at some point during the study period. Seventeen of them (12%) developed hypomania and three (2%), one in each treatment arm, developed severe hypomania. No participant switched to a manic episode or was hospitalized for switching to hypomania.
Using the survival models, this corresponded to an estimated 16-week switch rate of 17.9% overall (19.4% for lithium, 19.9% for sertraline, and 13.4% for the combination treatment) after accounting for early study discontinuation. There was no evidence of a treatment group difference in rates of switching based either on the raw estimates or after covariate adjustment. There was also no evidence of a group-by-rapid cycling interaction for switch rates. Most switches occurred relatively early (
Figure 1B). Of the 20 participants who experienced a switch, 11 (55%) switched within the first 4 weeks of treatment and 15 (75%) within the first 5 weeks.
Treatment Response Rates
Descriptive statistics for treatment response by medication group are presented in
Table 2, and the corresponding Kaplan-Meier curves in
Figure 1C. Eighty-nine participants (62.7%) met response criteria at some point during the study period. The treatment groups did not differ significantly in rate of or time to response in the raw survival curves, although the monotherapy groups performed nonsignificantly better than the combination therapy (log-rank test χ
2=4.76, df=2, p=0.093). There was no evidence of an accelerated response in the combination therapy group compared with the monotherapy groups. In the Cox model, there was a significant treatment group-by-rapid cycling interaction (χ
2=6.13, df=2, p=0.047) adjusting for the other covariates. Response rates were similar across treatment groups among participants with a rapid cycling course, but response rates were different across groups for those without a rapid cycling course. For non–rapid cyclers, response rates were superior in the monotherapy groups compared with the combination therapy group (
Figure 1D,
1E).
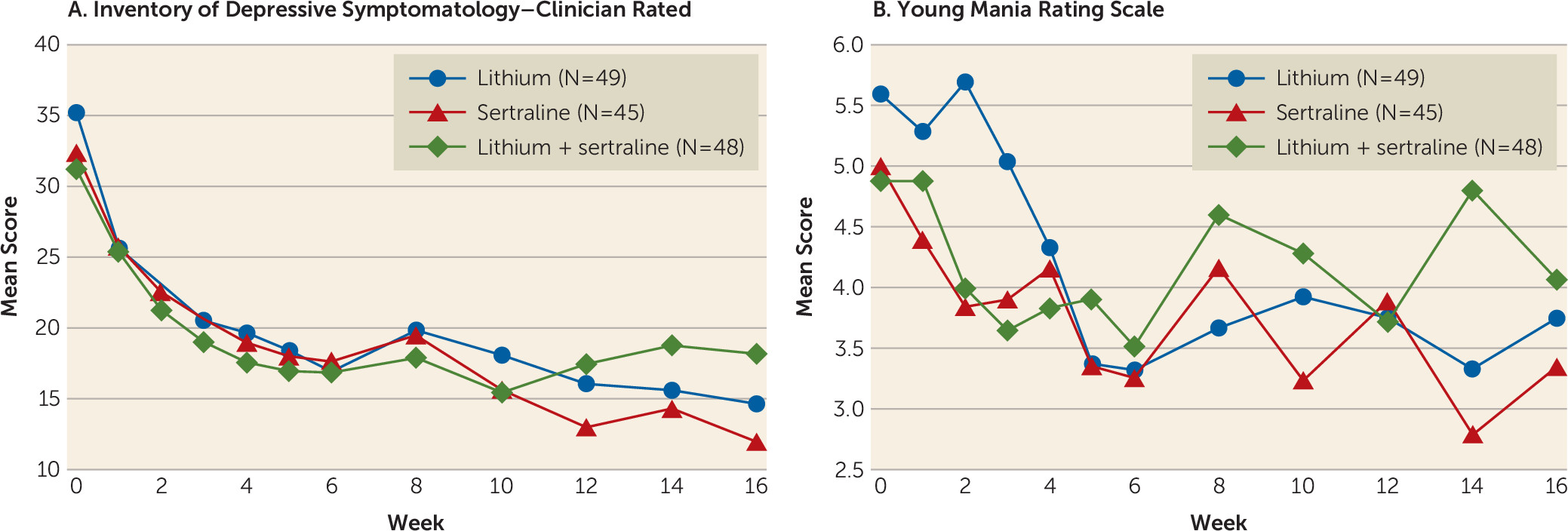
Plots of average weekly IDS-C and YMRS scores by treatment arm are presented in
Figure 2. Significant improvement in depressive symptoms was seen in all three treatments over the study period, with a sharper initial improvement over the first 6 weeks followed by a leveling off over the remainder of the treatment period (all p values, <0.001). In the model for depressive symptoms, there was a significant group-by-time interaction corresponding to some continued improvement in the monotherapy groups, but a leveling off in the combination therapy group (p=0.001).
Relationship of lithium serum levels to switching and treatment response.
Switch:
Participants receiving lithium (from either lithium group) who switched to hypomania had significantly lower mean serum lithium levels (0.41 mEq/L) than those who did not switch (0.63 mEq/L) (F=4.60, df=1, 128, p=0.034).
Treatment response:
Overall, there were no significant differences in lithium serum levels by treatment group or treatment response after covariate adjustment (
Table 3). However, the best estimates of the levels for the responders (0.61 mEq/L) were higher (i.e., in the expected direction) than those of the nonresponders (0.51 mEq/L).
Relationship of sertraline peak dosage to switching and treatment response.
Switch:
In the two groups that received sertraline, there was no significant difference in the proportion of participants reaching the minimum target dosage of 100 mg/day by switching status.
Treatment response:
There was also no difference in peak sertraline dosage by treatment group. However, participants who were treatment responders were significantly more likely to reach or exceed a peak dosage of 100 mg than were nonresponders after covariate adjustment (χ2=13.77, df=1, p<0.001; 50/55 [90.9%] among responders and 18/36 [50.0%] among nonresponders).
Relationship of a History of Substance Abuse to Switch, Dropout, and Treatment Response
Of the 140 participants for whom interview data were available on any history of substance abuse or dependence, 48 (28.6%) had a history of alcohol abuse or dependence, 38 (27.1%) had a history of drug abuse or dependence, and 31 (22.1%) had both. Among those with a history of drug abuse or dependence, 19 involved cannabis, 18 involved stimulants, and 15 involved other drugs, with many participants endorsing multiple types.
For our primary outcome, switch to hypomania, participants with a history of drug abuse or dependence were significantly more likely to experience a switch (χ2=12.82, df=1, p<0.001) after adjusting for treatment arm and the core set of covariates. Further analysis suggested that the effect of drug history on switching was driven largely by stimulant abuse or dependence (χ2=13.59, df=1, p<0.001). A history of alcohol abuse or dependence was not significantly associated with switching. For dropout, the pattern was similar, with no evidence for an effect of alcohol abuse or dependence but an effect that fell short of significance for a negative impact of drug history (χ2=4.01, df=1, p=0.055); there was no indication that this association was driven by a specific class of drugs. Finally, for participants across all three arms of the study who met the treatment response criteria, there was no indication of an effect of a history of drug abuse or dependence above and beyond the effect of other covariates. However, in all three treatment arms, those who had a history of alcohol abuse or dependence were significantly less likely to respond, all else being equal (χ2=6.43, df=1, p=0.012).
Relative Tolerability
There was no difference in the overall likelihood of developing a treatment-emergent side effect across the treatment arms after adjusting for the baseline covariates (see Table S3 in the online data supplement). While dropout due to medication side effects did not differ between groups, overall dropout rate was significantly greater in the combination treatment group.
Discussion
To our knowledge, this is the largest prospective randomized double-blind trial assessing comparative rates for switch, side effects, and treatment response of lithium monotherapy, SSRI monotherapy, and lithium/SSRI combination therapy for the acute treatment of bipolar II depression. We found no difference in risk of hypomanic switch across sertraline monotherapy, lithium monotherapy, or combination therapy. No participant had a manic switch. We also found no significant differences in response rates between treatment groups, and no acceleration effect when we compared combination therapy and monotherapy. We did, however, observe a significantly greater overall dropout rate in the combination therapy group.
The rates of switching to hypomania were modest but clinically important, as they ranged from 13% to 20%, depending on the method of calculation. The fraction of study enrollees who experienced an actual switch during the protocol provides one metric, while we also estimated switch rates over time (based on survival models that take dropout into account). The actual switch rates were 14.3%, 17.8%, and 10.4%, respectively, for the lithium, sertraline, and combination therapy groups. The estimated (Kaplan-Meier based) switch rates at study end were 19.4%, 19.9%, and 13.4%, respectively.
The switch rates in the present study comport with other open (
34,
35) and double-blind (
21) acute treatment trials that report low raw switch percentages, from 6.8% to 13.5%, in participants with bipolar II depression on SSRI monotherapy. Additionally, in our study, switch rates did not differ between rapid cyclers and non–rapid cyclers, which is consistent with two recent studies of patients with bipolar II depression who received monotherapy with an SSRI or a serotonin-norepinephrine reuptake inhibitor (
36,
37). That most switches in our study occurred within the first 5 weeks of treatment suggests that clinicians should monitor patients closely during this period.
We also note that our data suggest that individuals with bipolar II disorder have high rates of rapid cycling. To our knowledge, the rate of rapid cycling in the bipolar II population has not been well characterized and is an area where further exploration is needed. Our criteria for hypomanic switch were liberal (we used a low rating scale cutoff score for defining switch), so our rates may be somewhat higher than some other reports in the literature.
For participants on lithium, average serum lithium levels were significantly lower in those who switched (0.4 mEq/L) compared with those who did not (0.6 mEq/L). This suggests that either 1) for bipolar II patients treated for depression, lithium levels ≥0.6 may offer greater protection against switching or 2) those most at risk for switching may be unable to tolerate a sufficiently higher dosage. Our study was not designed to adequately disentangle these possibilities. The association of a lower lithium level with a lower threshold for switch or relapse is consistent with the literature on patients with bipolar I disorder. For participants on sertraline, the majority were taking dosages of 100 mg/day or higher. There was no relationship between dosage and switching, but a significant relationship was seen between dosage and meeting response criteria (see below). As noted above, the study was not designed to address dosage effects of sertraline, and further study is needed to sort out these issues.
For switch to hypomania or severe hypomania, we observed a significant effect of history of drug abuse or dependence, but not alcohol abuse or dependence, with participants who had a drug use history being more likely to experience a switch across all three treatment groups. This effect was driven largely by stimulant abuse or dependence, which was a highly significant finding. Consistent with our findings, Ostacher et al. (
38) found an association between substance abuse or dependence and mood switch in the Systematic Treatment Enhancement Program for Bipolar Disorder data set. The complex relationship between mood symptoms and substance use disorders is an area requiring additional research.
Treatment Response Rates
Our study was not powered to demonstrate what would be expected to be small differences in response rates between active treatments. Furthermore, as we did not have a placebo arm, it is not clear whether any of the three treatments would be superior to placebo. However, response rates across treatment groups were high, with 62.7% of participants maintaining our IDS-C and CGI-BP criteria for 2 consecutive weeks during the study period and 57% meeting criteria at study exit. These findings are consistent with most open and blind treatment trials of bipolar II depression with monotherapy with either lithium (
39) or an SSRI (
34,
37,
39,
40). As there was a higher dropout rate in the lithium/sertraline group with no significant response advantage, combination therapy may be the least desirable option for short-term treatment. However, a significant dosage relationship was noted for participants on the higher sertraline dosages. Of interest, the pattern of treatment response differed between rapid and non–rapid cyclers: the rapid cyclers showed no difference across the three regimens, whereas the non–rapid cyclers had a significantly lower response to combination treatment.
Overall, our participants with bipolar II disorder were able to achieve what would be considered a low therapeutic serum lithium level range for bipolar I patients, with a (nonsignificantly) higher average serum level among responders (0.62 mEq/L) than nonresponders (0.51 mEq/L). It is possible that bipolar II patients respond at lower serum levels than bipolar I patients; however, our study was not designed to evaluate relationships between serum lithium levels and treatment response.
Tolerability
No significant between-group differences were found in the likelihood of developing a treatment-emergent side effect, but the overall dropout rate was significantly higher in the lithium/sertraline combination arm, the treatment most commonly recommended in clinical practice. These results suggest that monotherapy in general, and sertraline monotherapy in particular, may be a viable option for some patients, which is of significant clinical import to patients and their physicians.
Implications for Practice
There are currently few treatment guidelines specifically aimed at the treatment of patients with bipolar II disorder. Virtually all treatment guidelines are oriented toward patients with bipolar I disorder, in whom the majority of well-controlled studies have been conducted. This was noted in the recent Florida Medicaid Guidelines (
41). However, in the 2013 Canadian Network for Mood and Anxiety Treatments (CANMAT) guidelines (
42), there is a separate section addressing the treatment of patients with bipolar II disorder. The results from the present study suggest that antidepressant monotherapy should be reevaluated in CANMAT as its current third-line treatment recommendation for bipolar II depression. While our study lacked a placebo arm to support placing this recommendation as a first-line therapy, our results support the possibility that, unlike in bipolar I patients, an antidepressant monotherapy may be appropriate and carry few risks in some patients with bipolar II disorder.
Limitations
Our study has several limitations. Because we excluded participants with mixed depression, active substance abuse, or previous nonresponse to adequate trials of sertraline or lithium, the generalizability of our results is limited. Our sample also may not be representative with respect to rapid cycling. However, this does not affect any of our primary results, and, moreover, because of the nature of rapid cycling, we note that even if we had not taken this factor into account in our models, it would have been more likely to bias estimates in the direction of even higher switch rates than those we found across treatment groups.
Because our study was not designed with a placebo arm, we can neither affirm antidepressant efficacy or superiority of one drug over another nor be certain of whether switch rates are attributable to treatment or natural course of illness. Also, there was no augmentation therapy arm. We therefore cannot draw conclusions about either the addition of lithium to an antidepressant or the reverse in the treatment of bipolar II depression.
Despite having the largest sample size to date in this area, our study was only powered to detect a large difference in switch rates, and thus it is possible that a
smaller difference in switch rates exists between treatment groups. If a smaller difference was missed by our study, the clinical significance of such a differential treatment-related risk for switch would be questionable. With our sample of 142 patients, we had an 80% power to detect large differences in switch rates between antidepressant monotherapy and the other two groups in survival models (hazard rate corresponding to 28% versus 6% switching after 16 weeks, based on the literature, allowing for 50% dropout). However, the observed differences in switch rates were smaller than anticipated (see
Figure 1B). Furthermore, at 16 weeks, our study was too brief to definitively address risk for cycle acceleration with antidepressant monotherapy. However, two long-term follow-up studies of patients with bipolar II depression (
40,
43), involving double-blind monotherapy with fluoxetine, lithium, or placebo for 26 or 50 weeks, found no pattern of increased cycling in the fluoxetine monotherapy group compared with the other groups, although this observation was made in an enriched sample of fluoxetine responders. Finally, our study was neither designed nor powered to make definitive determinations about relationships between lithium dosage or serum levels and clinical response or switch rates.