Single modality structural and functional imaging studies in those who attempt suicide implicate structure-function relationships, suggesting a need for multimodal studies. Few studies have examined associations of imaging measures with suicide-related symptoms and behaviors, such as attempt lethality, ideation, intent, impulsivity, and hopelessness. In adults who have attempted suicide, ventral prefrontal volume decreases and dysfunction have been linked to lethality (
7,
8), frontal dysfunction and white matter abnormalities to ideation (
9,
10), and orbitofrontal white matter fractional anisotropy decreases to impulsivity in bipolar disorder (
11). Imaging studies of suicidal ideation are rare (
1); one study of ideation in pediatric epilepsy showed decreased orbitofrontal white matter (
12).
This study of bipolar disorder in adolescents and young adults who have attempted suicide, compared with those who have not, combines sMRI, DTI, and fMRI connectivity methods to study gray matter volume, white matter integrity, and functional connectivity. We hypothesized that participants with a history of suicide attempt would show lower ventral frontal and mesial temporal gray matter, and lower white matter fractional anisotropy and functional connectivity in their connections. We explored relationships between imaging findings across modalities and with attempt lethality, anticipating ventral prefrontal associations, and with suicidal ideation and intent, hopelessness, and impulsivity. We explored comparisons with a healthy adolescent and young adult group.
Discussion
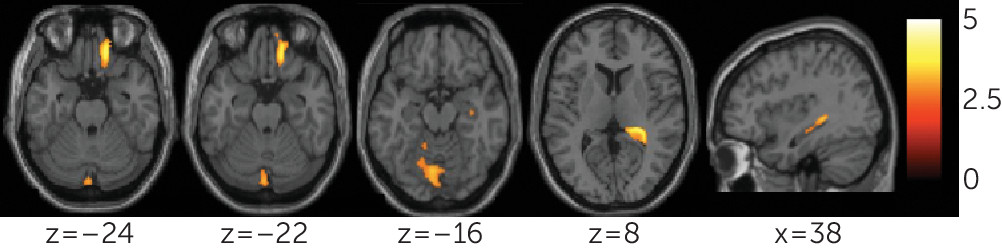
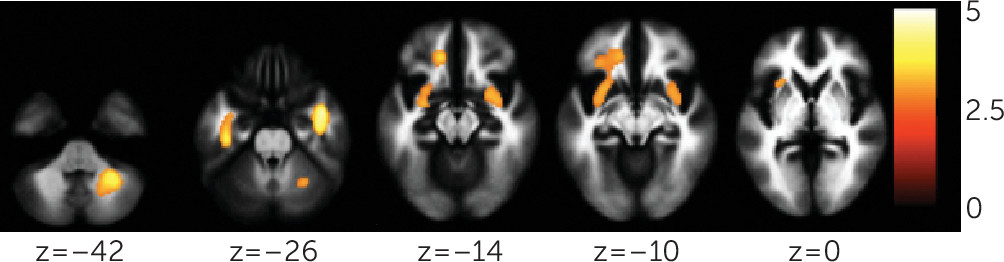
This study demonstrated reductions in gray matter volume and integrity of structural and functional connections in a frontolimbic system in a sample of individuals with bipolar disorder who have attempted suicide, compared with nonattempters. Decreases were observed in gray matter volumes in the right orbitofrontal cortex and hippocampus, and bilateral cerebellum. Diminished integrity of white matter in the uncinate and ventral frontal and right cerebellum regions, and decreased functional connectivity between the amygdala and left ventral prefrontal and right rostral prefrontal regions, were also observed. Cerebellar gray matter volume was associated with uncinate and cerebellar fractional anisotropy, and orbitofrontal gray matter volume was associated with cerebellar fractional anisotropy. Among attempters, decreases in rostral and ventral prefrontal functional connectivity were associated with severity of suicidal ideation and attempt lethality, respectively.
Gray matter volume reductions associated with suicide attempts in bipolar disorder were in the orbitofrontal cortex, hippocampus, and cerebellum, regions implicated in emotion processing and regulation and in bipolar disorder (
27). The orbitofrontal cortex plays a central role in regulating emotion responses, including through its connections to the amygdala, hippocampus, and cerebellum (
27). Volume decreases in the orbitofrontal cortex have been reported in adult attempters with major depressive disorder (
28). The hippocampus may also influence emotion reactions and regulatory processes, given its role in encoding and recall of the emotional significance of events. Moreover, impaired memory processes have been associated with suicide attempts, implicating the hippocampus (
29). Cerebellar connections with the prefrontal cortex and amygdala suggest a role for the cerebellum in emotion regulation, consistent with emotion dysregulation observed with cerebellum lesions (
27). Hippocampus and cerebellum volume decreases have been observed in adult attempters (
30,
31) but have been studied primarily in attempters with major depression, and some findings are inconsistent (
28,
32). We did not detect differences in amygdala volume between the bipolar disorder attempters and nonattempters. Varying findings may be attributed to differences in imaging methodology and subject samples.
White matter findings were in regions of the bilateral uncinate, extending into left ventral frontal regions, and in the right cerebellum. The uncinate provides major connections between the ventral prefrontal cortex and amygdala and therefore is implicated in emotion regulation. In bipolar disorder, white matter orbitofrontal decreases have been reported and were associated with impulsivity (
11). The white matter decreases observed herein in attempters included more caudal frontal and temporal areas and were not associated with impulsivity. Right uncinate fractional anisotropy values were lower in association with rapid cycling, a potential risk factor for suicide in bipolar disorder (
33), suggesting a link to mood dysregulation. Decreased white matter volumes have been observed in the cerebellum in attempters with major depression (
31). The cerebellum finding in attempters with bipolar disorder suggests it may be a structural abnormality associated with attempts across these mood disorders.
Exploratory analyses also provided preliminary evidence for associations between some of the gray and white matter findings. These findings raise the possibility of interactions between the development of gray and white matter in suicide risk. We cannot address this possibility using this study’s data. However, reciprocal relationships between neurons and glia during development may contribute to concomitant gray and white matter developmental changes (
34–
36). It is also possible that gray and white matter associations are the results of other biological processes, as opposed to a direct interaction between their developmental processes. For example, they may reflect responses to common genetic or environmental factors.
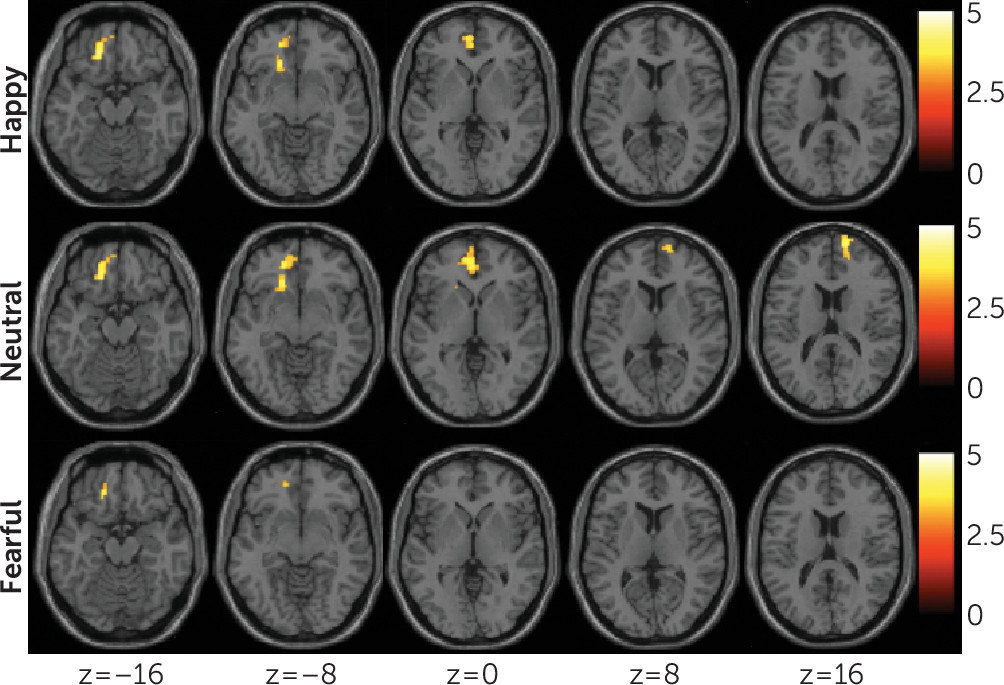
Functional connectivity between the amygdala and the ventral prefrontal cortex is critical in emotion regulation and showed significant decreases during the processing of happy and neutral faces, and decreases during the processing of fearful faces that did not reach significance, in attempters compared with nonattempters. Studies suggest emotion regulation deficits in adolescent suicide attempters, and adolescents may attempt suicide in an effort to dampen intolerable emotional states (
37). Moreover, adolescents with higher affect dysregulation are more likely to have multiple suicide attempts (
38). Previous fMRI studies have shown increased responses to negative emotional faces, specifically angry faces, in frontal circuitry in adolescents (
39), similar to adults (
2). Depression and suicidal ideation have been associated with altered responses to neutral face stimuli in adults, particularly with projecting a negative bias (
40). Together with significant reductions in functional connectivity between the amygdala and ventral prefrontal cortex in the happy condition—and in the fearful condition, although this did not reach significance—the observation regarding the processing of neutral face stimuli suggests that more widespread affective dysregulation may be present in suicide attempters with bipolar disorder, limiting modulation of emotional responses and impairing effective management of affect.
Decreased functional connectivity from the amygdala was also noted in the right rostral prefrontal cortex in the neutral condition, suggested by exploratory analyses to be associated with severity of suicidal ideation. Less is known about the role of the rostral prefrontal cortex in suicidal behavior. The experience of mental pain in attempters during recall of suicidal episodes showed associations with decreased functioning of the rostral prefrontal cortex (
41), a region implicated in self-referential and introspective mental functions (
42) altered in suicidal ideation and during suicidal acts (
43). Frontal dysfunction was observed in a study of adults with suicidal ideation (
9). In depressed youths, maladaptive emotion regulation response tendencies increased the likelihood of suicidal ideation, planning, and action, while adaptive regulatory responses decreased suicidal behavior (
44). Because the rostral prefrontal cortex is important in the development of regulation of emotions and adaptive response tendencies (
45), it is possible that emotion dysregulation, resulting from disruptions in prefrontal system functional connectivity, contributes to suicidal ideation and risk for suicidal behavior.
The magnitude of functional connectivity between the amygdala and the left ventral prefrontal cortex was negatively associated with attempt lethality. Individuals who make high-lethality attempts may be most at risk for suicide death (
8). Our finding comports with adult neuroimaging studies demonstrating associations between attempt lethality and ventral prefrontal hypometabolism (
8). High lethality has been associated with highly planned, less impulsive attempts (
8). Together with the lack of associations between impulsivity and reductions in structural and functional integrity among attempters in this study, this finding suggests that altered ventral prefrontal functioning in attempt lethality may not, at least for some individuals, be related to its role in controlling impulses but may relate to emotion dysregulation. However, impulsivity was assessed at scanning. It is possible that associations with ventral prefrontal alterations may exist at the time of attempt.
For most regions in which attempters differed from nonattempters, attempters, but not nonattempters, also differed significantly from healthy comparison subjects. Although this suggests that the findings may be related to suicidal tendencies, comparisons with individuals with other disorders are needed. It is also possible that the findings are a consequence of bipolar disorder or of suicide attempts. Future longitudinal studies are needed to elucidate the predictive value of these results. Orbitofrontal and cerebellar gray matter volume decreases in healthy comparison subjects relative to suicide attempters did not reach significance. This suggests that gray matter volumes in these regions may better differentiate attempter status within bipolar disorder. Orbitofrontal volume decreases in attempters compared with nonattempters have been reported previously in bipolar disorder (
46). Orbitofrontal decreases have also been reported, relative to healthy comparison groups, in attempter groups with major depressive disorder and borderline personality disorder (
5). It is possible that aspects of our sample or methods decreased the ability to detect significant differences between attempters and healthy comparison subjects. Cerebellar findings are mixed (
5), and additional studies are warranted.
Study limitations include the modest sample size. While no effects in the regions of group differences were detected for gender, medication, substance comorbidity, mood state, or psychosis, the number of participants in the subgroups for the factors assessed was small, limiting power. There were not sufficient numbers of subjects to perform meaningful analyses for subtypes of medications and nonsubstance psychiatric comorbidities. Age range was large, and the sample included pubertal teenagers and young adults. While age was included as a covariate, developmental brain changes could still have affected results. Although suicide attempt and ideation history were assessed systematically, assessments were based on retrospective subject reporting, which could decrease accuracy. Results were reported with a primary threshold of p<0.005, with AlphaSim spatial extent thresholding (p<0.05, corrected), in order to balance the effort to avoid premature strict correction that may limit report of findings that could prove useful in the future to the field, especially as there are rare similar data in the relatively young field of suicide research, with potential report of spurious results. However, caution should be taken in considering the results because lower primary thresholds may lead to spurious findings when utilizing cluster-extent-based thresholds (
47). We noted which results survived to a threshold of p<0.001, with and without AlphaSim spatial extent thresholding (p<0.05, corrected), to provide this further information. Correlational analyses should be considered preliminary. Exploratory correlational analyses were not controlled for multiple comparisons. Some associations across imaging modalities and with behaviors were in directions anticipated but did not reach significance, potentially owing to the sample size. Directionality of the DTI acquisition was limited. Additional analyses (e.g., analyses of group differences in activation and/or functional connectivity analyses using psychophysiological interaction analysis methods to further isolate stimulus-related connectivity) could aid in further interpretation of the data. However, as in the previous published work of our group (
20,
22) and of other research groups, there can be complex interactions between mood state and the valence of emotional stimuli. Thus, the sample sizes in the present study limit the ability to fully assess further interactions with suicide history, and additional comprehensive analyses are beyond the scope of this article.
In summary, this multimodal structural and functional neuroimaging study of adolescent and young adult suicide attempters with bipolar disorder demonstrates decreases in gray matter and white matter structural and functional connectivity in a ventral frontolimbic neural system that subserves emotion regulation. This study provides preliminary evidence for gray matter and white matter relationships and associations of system features with suicidal ideation and attempt lethality. Integrated study of brain structure and function, and their associations with symptoms and behavior, in suicide research is important in elucidating risk mechanisms that may involve complex, parallel, and interacting relationships between developing circuitry and symptoms and behaviors. Longitudinal studies are needed to more fully investigate development of system features, their role in the transition from suicidal ideation to behavior and risk for completed suicide, and the role of emotion regulation in suicidal behavior in bipolar disorder and whether it generalizes to other disorders.