Can Botulinum Toxin Help Patients With Borderline Personality Disorder?
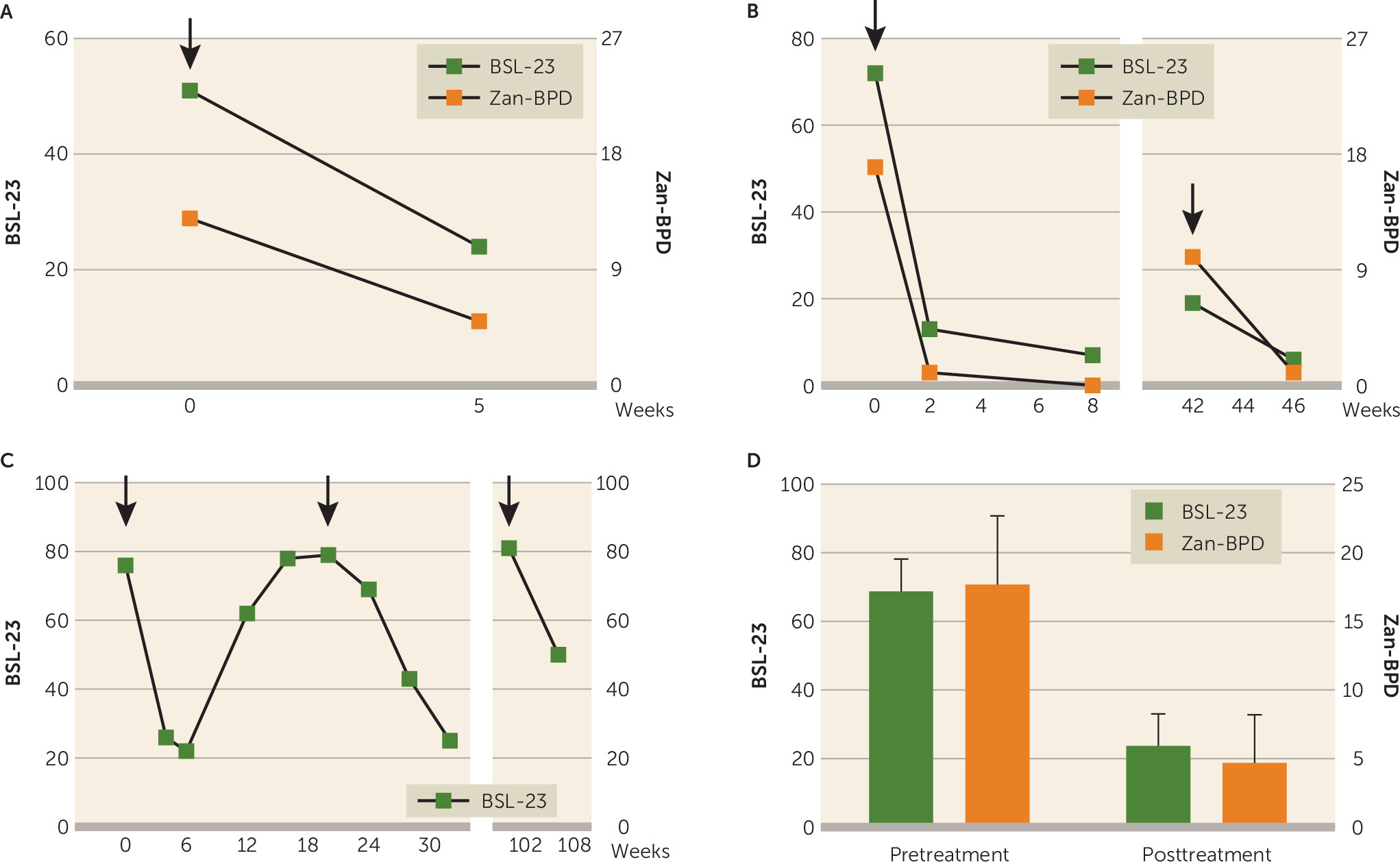
Female patients ages 20–59 years with a diagnosis of borderline personality disorder received botulinum toxin treatment with 29 U of onabotulinumtoxinA at five injection sites in the glabellar region during inpatient treatment between December 2012 and July 2015 at the Hannover Medical School (N=5) and at the Asklepios Clinic North–Ochsenzoll (N=1). In all six patients, previous and current pharmacological (antidepressants, antipsychotics) and psychotherapeutic (including dialectic behavioral therapy) treatment attempts had been insufficient. Two to 6 weeks following the injection of botulinum toxin, the symptoms of borderline personality disorder as measured by the Zanarini borderline personality disorder rating scale and/or the Borderline Symptom List had improved by 49%−94% from baseline values (Wilcoxon signed ranks test, p≤0.05; Figure 1). Patients also showed a reduction in impulsivity, self-harming behavior, agitation, and concomitant depressive symptoms as well as an improvement in social functioning. Four patients sought repetition of the treatment months later in an outpatient setting, which led to a replication of the clinical improvement.
Supplementary Material
- View/Download
- 99.99 KB
References
Information & Authors
Information
Published In
History
Keywords
Authors
Funding Information
Metrics & Citations
Metrics
Citations
Export Citations
If you have the appropriate software installed, you can download article citation data to the citation manager of your choice. Simply select your manager software from the list below and click Download.
For more information or tips please see 'Downloading to a citation manager' in the Help menu.
View Options
View options
PDF/EPUB
View PDF/EPUBLogin options
Already a subscriber? Access your subscription through your login credentials or your institution for full access to this article.
Personal login Institutional Login Open Athens loginNot a subscriber?
PsychiatryOnline subscription options offer access to the DSM-5-TR® library, books, journals, CME, and patient resources. This all-in-one virtual library provides psychiatrists and mental health professionals with key resources for diagnosis, treatment, research, and professional development.
Need more help? PsychiatryOnline Customer Service may be reached by emailing [email protected] or by calling 800-368-5777 (in the U.S.) or 703-907-7322 (outside the U.S.).

