Treatment-Resistant Schizophrenia: Treatment Response and Resistance in Psychosis (TRRIP) Working Group Consensus Guidelines on Diagnosis and Terminology
Abstract
Objective:
Method:
Results:
Conclusions:
| Requirements of Previous Treatment | ||||||
|---|---|---|---|---|---|---|
| Guideline | Minimum Number of Failed Antipsychotic Trials | Specified Antipsychotic | Adequate Treatment Episode Duration | Dosage | Severity of Illness | Other |
| APA (6) | 2 | “At least one of which is a second-generation antipsychotic” | ≥6 weeks | Therapeutic range | “A clinically inadequate response” “and for patients with persistent suicidal ideation or behavior that has not responded to other treatments” | |
| RANZCP (23) | 2 | Recommends both first and second trial to be of an atypical antipsychotic | 6–8 weeks | Specific dosages specified | “Poor response” | “If poor … adherence, or persistent suicide risk, positively offer trial of clozapine” |
| BAP (24) | 2 | “One of the trials should be of an antipsychotic with an established, favourable efficacy profile in comparison with other antipsychotics” | “Adequate” | “Adequate” | “Schizophrenic illness has shown a poor response to, or intolerance of the neurological side effects of [previous treatment]” | “Poor … adherence and … substance use should be excluded as causes of the … poor response to antipsychotic” |
| IPAP | 2 | “An atypical or, if not available, a trial of haloperidol, chlorpromazine or other typical antipsychotic” | 4–6 weeks | “Adequate” | “Psychosis or moderate-to-severe tardive dyskinesia or tardive dystonia after adjusting dose” | |
| Maudsley (25) | 2 | Consider use of either first- or second-generation antipsychotic | 2–3 weeks for trial of first antipsychotic in first-episode psychosis; 6-week trial for a subsequent antipsychotic before clozapine | At least minimum effective dose, then titrated to response | Not specified | |
| MOHS (26) | 2 | No | Adequate | Adequate | “Illness has not responded adequately to treatment” | Two trials should be given “sequentially” |
| NICE (5) | 2 | “One of the drugs should be a non-clozapine second-generation antipsychotic” | Not specified | Adequate | “Illness has not responded adequately to treatment” | Two trials should be given “sequentially” |
| WFSBP (7) | 2 | “One of which should be an atypical antipsychotic” | 6–8 weeks | Recommended dosage | No improvement at all or only insufficient improvement in the target symptoms | Adherence should be ensured, if necessary by checking drug concentrations |
General Requirements For Treatment Resistance
Method
TRRIP Meetings
Results
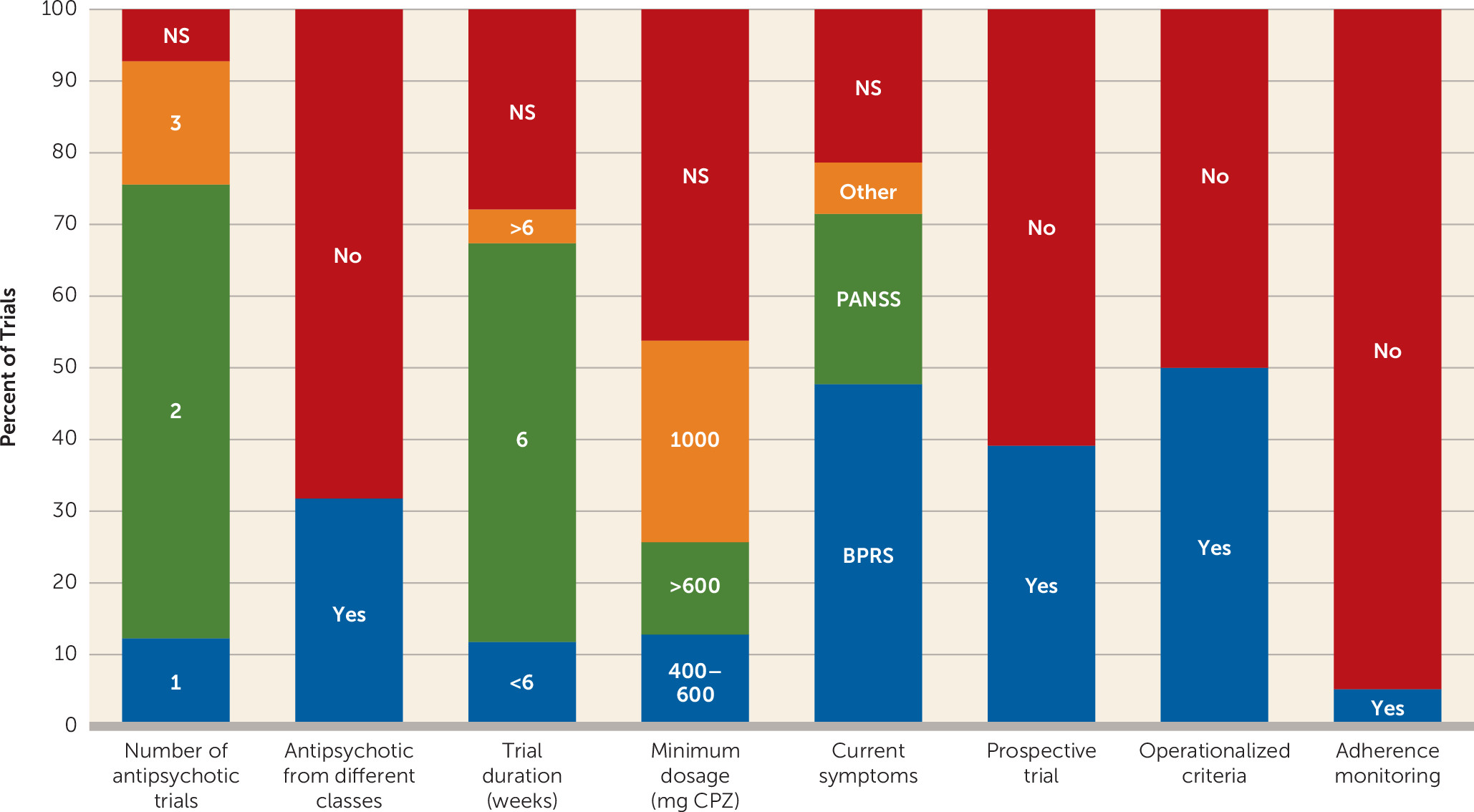
Systematic Review
Consensus Recommendations
| Domain and Subdomain | Minimum Requirement | Optimum Requirement |
|---|---|---|
| Current symptoms | ||
| Assessment | Interview using standardized rating scale (e.g., PANSS, BPRS, SANS, SAPS) | Prospective evaluation of treatment using a standardized rating scale |
| Severity | At least moderate severity | At least moderate severity and <20% symptom reduction during a prospective trial or observation of ≥6 weeks |
| Duration | ≥12 weeks | ≥12 weeks; specify duration of treatment resistance |
| Subjective distress | Not required | Not required |
| Functioning | At least moderate functional impairment measured using a validated scale (e.g., SOFAS) | At least moderate functional impairment, measured using a validated scale (e.g., SOFAS) |
| Adequate treatment | ||
| Assessment of past response | Information to be gathered from patient/carer reports, staff and case notes, pill counts, and dispensing charts | Information to be gathered from patient/carer reports, staff and case notes, pill counts, and dispensing charts |
| Duration | ≥6 weeks at a therapeutic dosage; record minimum and mean (SD) duration for each treatment episode | ≥6 weeks at a therapeutic dosage; record minimum and mean (SD) duration for each treatment episode |
| Dosage | Equivalent to ≥600 mg of chlorpromazine per day.b Record minimum and mean (SD) dosage for each drug. | Equivalent to ≥600 mg of chlorpromazine per day.b Record minimum and mean (SD) dosage for each drug. |
| Number of antipsychotics | ≥2 past adequate treatment episodes with different antipsychotic drugs. Specify median number of failed antipsychotic trials. | ≥2 past treatment episodes with different antipsychotic drugs and at least one utilizing a long-acting injectable antipsychotic (for at least 4 months). Specify median number of failed antipsychotic trials. |
| Current adherence | ≥80% of prescribed doses taken. Adherence should be assessed using at least two sources (pill counts, dispensing chart reviews, and patient/carer report). Antipsychotic plasma levels monitored on at least one occasion. Specify methods used to establish adherence. | Same as the minimum criteria, with the addition of trough antipsychotic serum levels measured on at least two occasions separated by at least 2 weeks (without prior notification of patient) |
| Symptom domain | Positive, negative, cognitive | Same as for minimum criteria |
| Time course | Early onset (within 1 year of treatment onset), medium-term onset (1–5 years after treatment onset), late onset (>5 years after treatment onset) | Same as for minimum criteria |
| Ultra–treatment resistant: clozapine | Meets the above criteria for treatment resistance plus failure to respond to adequate clozapine treatmentc | Same as for minimum criteria |
1. Terminology.
2. Clinical subspecifiers.
3. Symptom thresholds.
3.1. Rating scales.
3.2. Absolute thresholds.
3.3. Symptom change.
3.4. Functional impact.
4. Characterizing treatment resistance.
4.1. Degree.
4.2. Temporal development.
5. Defining adequate treatment.
5.1. Duration.
5.2. Dosage.
5.3. Number of past treatment episodes.
5.4. Clozapine-resistant schizophrenia.
5.5. Adherence.
6. Defining adequate treatment responders.
| Measure | Criterion |
|---|---|
| Symptom severity | Symptoms rated at no more than mild severity |
| Duration | Response sustained for a minimum of 12 weeks |
| Functioning | Impairment rated as mild or better on a standardized scale (e.g., SOFAS) |
Discussion
Strengths and Limitations
Conclusions And Future Directions
Footnotes
Supplementary Material
- View/Download
- 285.93 KB
References
Information & Authors
Information
Published In
History
Keywords
Authors
Author Contributions
Funding Information
Metrics & Citations
Metrics
Citations
Export Citations
If you have the appropriate software installed, you can download article citation data to the citation manager of your choice. Simply select your manager software from the list below and click Download.
For more information or tips please see 'Downloading to a citation manager' in the Help menu.
View Options
View options
PDF/EPUB
View PDF/EPUBLogin options
Already a subscriber? Access your subscription through your login credentials or your institution for full access to this article.
Personal login Institutional Login Open Athens loginNot a subscriber?
PsychiatryOnline subscription options offer access to the DSM-5-TR® library, books, journals, CME, and patient resources. This all-in-one virtual library provides psychiatrists and mental health professionals with key resources for diagnosis, treatment, research, and professional development.
Need more help? PsychiatryOnline Customer Service may be reached by emailing [email protected] or by calling 800-368-5777 (in the U.S.) or 703-907-7322 (outside the U.S.).

