The syndrome of major depressive disorder, a highly heterogeneous clinical condition, has largely defied meaningful subtyping (
1). First-line treatments for major depression include an evidence-based psychotherapy, such as cognitive-behavioral therapy (CBT), or antidepressant medication (
2). Both treatments have roughly equivalent efficacy, on average, for outpatients with major depression, though the remission rates of 30%−40% with either treatment alone are low (
2). Combination treatment with psychotherapy and antidepressant medication improves remission rates, but barriers such as cost, time, and patient preference preclude this option for many patients (
3). Importantly, some patients who do not respond to one treatment intervention exhibit an excellent response when switched to the alternative (
4,
5). This observation strongly suggests that biological or psychological variability may be identified and, thereby, improve the precision of treatment selection for individual depressed patients (
6).
Despite extensive efforts, work to identify clinical predictors of outcomes to treatments in nonpsychotic major depression has been disappointing. Depressive symptom severity has received the most attention among the clinical predictors, but the largest patient-level meta-analyses found no difference in outcome among patients treated with CBT or antidepressant medication based on severity (
7) or depressive clinical subtype (
8). The consistent failure of clinical features meaningfully to inform treatment selection serves as an impetus to identify biomarkers predictive of treatment outcomes (
9,
10). Unfortunately, genetic testing, neuroimaging, and psychophysiological approaches, although promising, have not yet proven sufficiently accurate or replicable to warrant clinical application to individual patients (
11).
Neuroimaging using positron emission tomography (PET) or functional MRI (fMRI) has been used extensively to characterize brain states of depressed patients. Among patients with major depression compared with healthy control subjects, relative hyperactivity of limbic brain regions, including the amygdala, insula, and subcallosal cingulate cortex (SCC), is consistently reported; hypoactivity in the dorsolateral prefrontal cortex is another replicated finding (
12). However, average differences between groups may mask important heterogeneity between individuals (
13), with some patients not showing these changes, or even demonstrating opposite patterns (e.g., increased metabolism in the dorsolateral prefrontal cortex) (
14). This variability in brain states across patients likely has important implications for treatment responsiveness.
Optimal application of precision medicine in depression should involve the prediction of both the desired outcome (remission) and the most undesired outcome (treatment failure). Remission is the goal of treatment because long-term wellness and overall functioning are greater among patients who fully remit from treatment compared with those who respond to a lesser degree or show no response (
2). However, avoiding treatment failure is also a vitally important outcome (
24). Because treatment efficacy can only be known after 6–12 weeks of treatment, application of an ineffective treatment prolongs patient suffering and role dysfunction, potentially increasing feelings of hopelessness and interpersonal strife, with persistence of suicidal ideation. These severe consequences from choosing the “wrong” treatment for a patient underscore the need for biomarkers predictive of both remission and treatment failure (
25). Furthermore, because combination treatment with both psychotherapy and medication for major depression is often not required or not feasible, selection of the initial treatment is typically a forced choice between psychotherapy and medication (
5). An optimal biomarker could identify whether failure to improve with one treatment modality could simultaneously predict improvement with the alternative modality.
Recently, resting-state metabolic activity assessed by [
18F]-fluorodeoxyglucose (FDG) PET found six brain regions that were differentially associated with the outcomes of remission and treatment failure among major depression patients randomly assigned to treatment with CBT or escitalopram. Metabolic activity in the right anterior insula emerged as the best candidate for use as a treatment selection biomarker (
26), with support provided by the finding that, among nonremitters to monotherapy, the same biomarker predicted eventual remission after addition of the alternative treatment (
27). Due to the cost and radiation exposure involved in PET imaging, more readily available and less expensive fMRI methods have appeal as an alternative approach for assessing regional brain activity. Resting-state functional connectivity is an fMRI technique that measures the degree to which separate brain regions demonstrate temporal correlations in the low-frequency components of the blood-oxygen-level dependent signal. The resting-state functional connectivity signal has identified brain networks involved in several aspects of mental functioning in healthy subjects, and resting-state functional connectivity in these networks differed between healthy control subjects and major depression patients in several studies (
28).
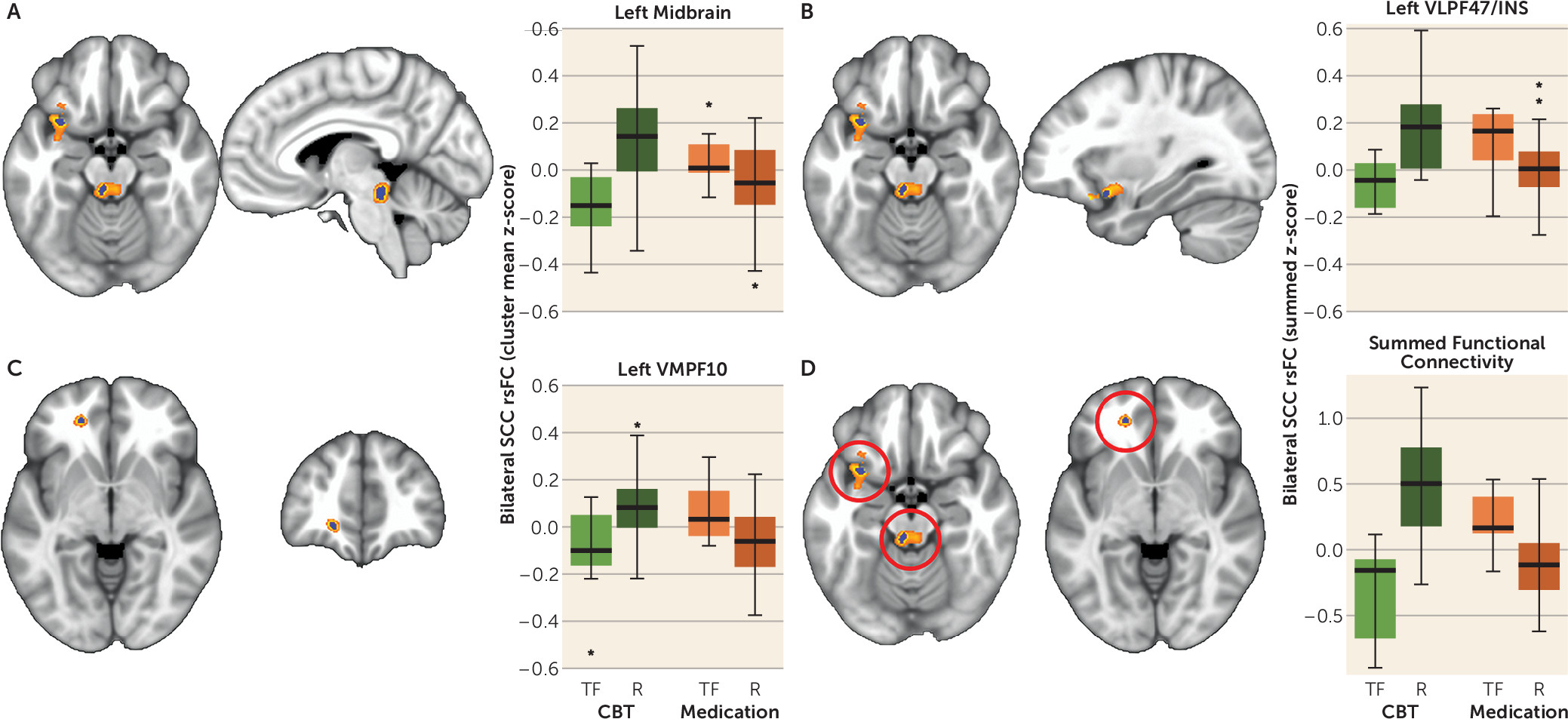
Of the many important frontal and limbic regions identified using fMRI studies of major depression, activity in the SCC has consistently emerged as a core component of major depression pathophysiology (
29). The SCC is an extensively connected component of the limbic system that modulates emotional behavior and is particularly involved in feelings of sadness (
30,
31). Greater functional connectivity between the SCC and the default mode network is present in patients with treatment-resistant depression (
32). Elevated pretreatment SCC metabolism has been associated with poorer outcomes to treatment with antidepressant medication (
33–
35), CBT (
17,
35), and the combination of antidepressant medication and CBT (
36). Finally, deep brain stimulation to the SCC and its cortical and subcortical connections may be efficacious in patients with highly treatment-resistant depression (
37,
38).
Discussion
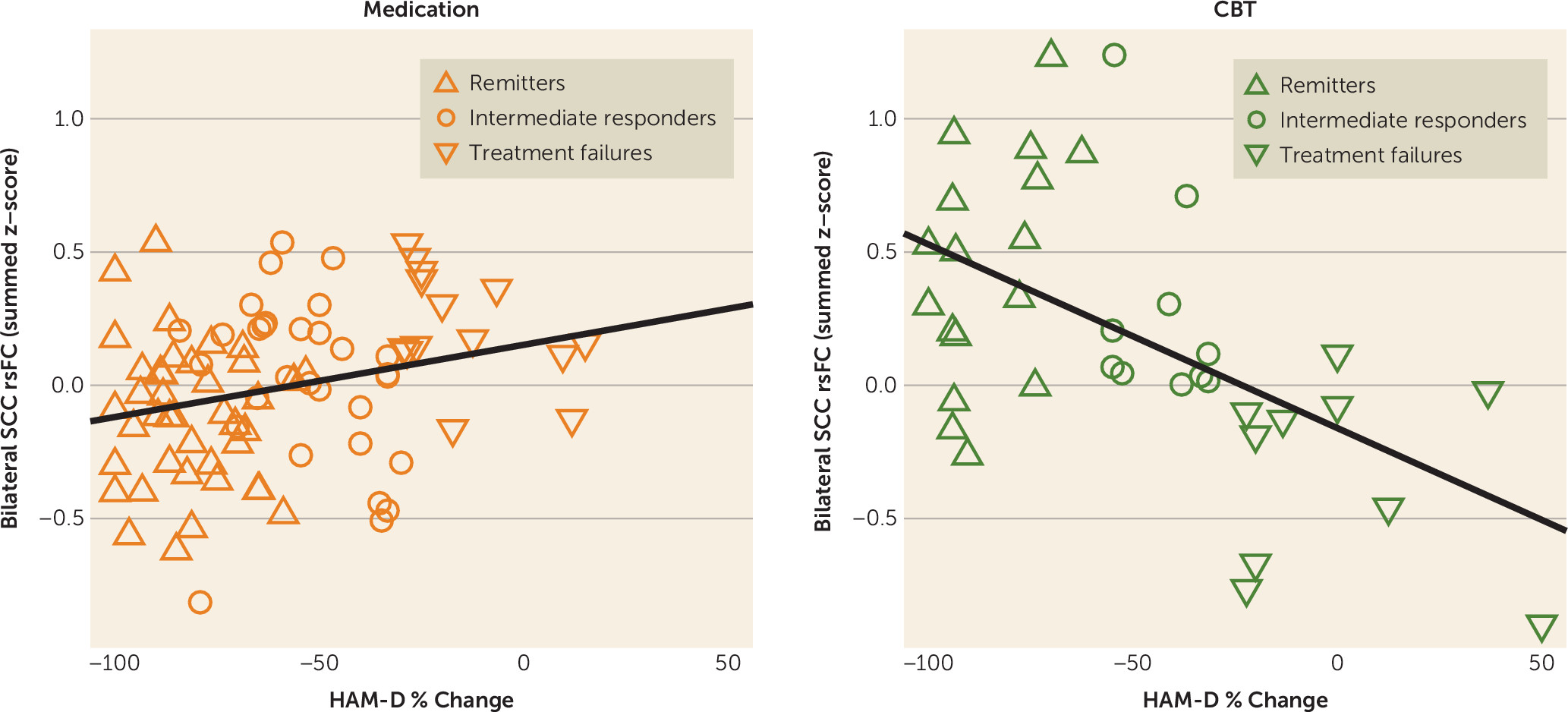
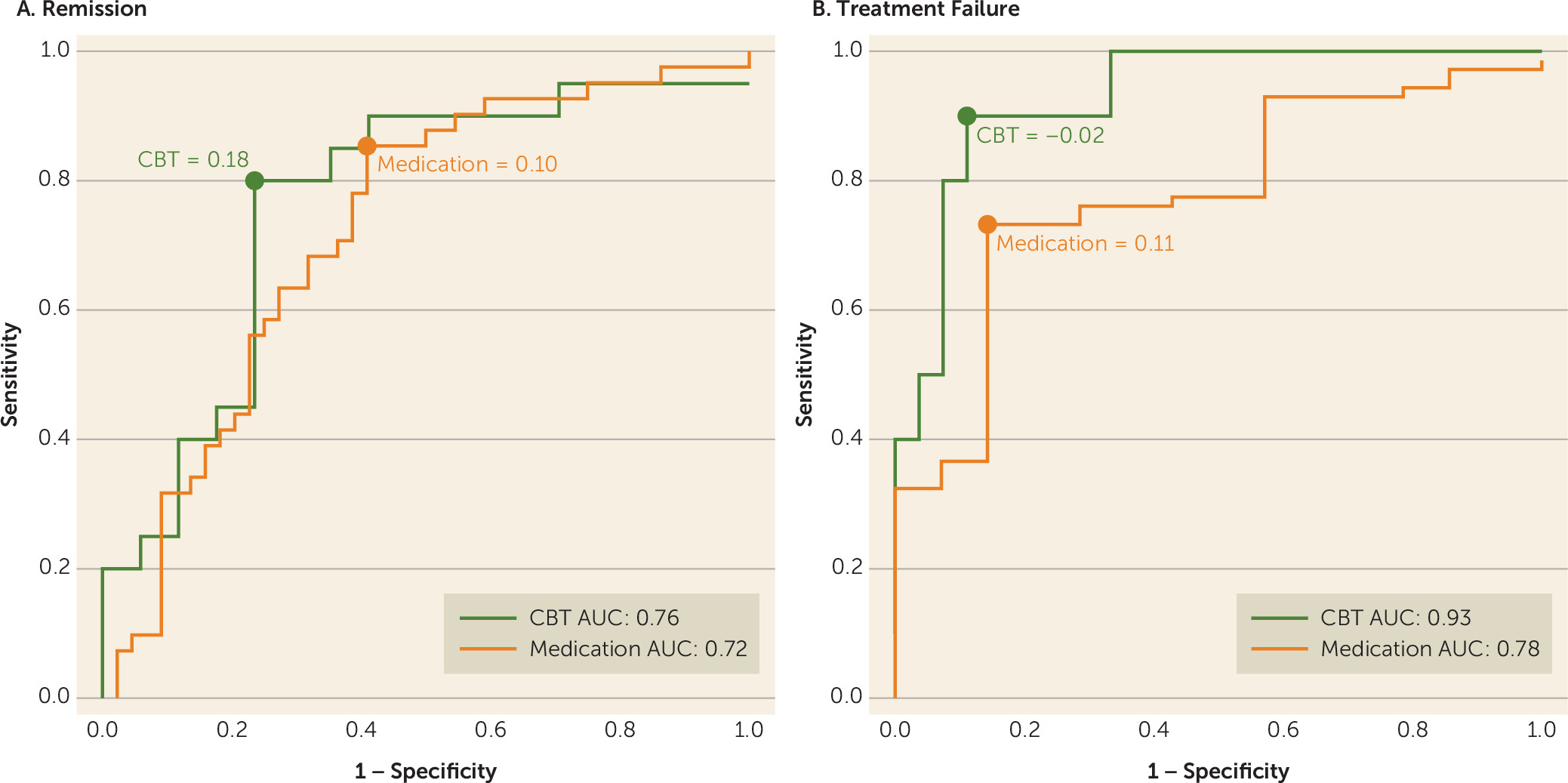
In this study of previously untreated adults with major depression, outcomes after 12 weeks of treatment with randomly assigned medication or CBT were associated with the degree of resting-state functional connectivity between brain regions involved in mood regulation—specifically, the SCC and 1) the left frontal operculum (incorporating the BA47 in the ventrolateral prefrontal cortex [VLPFC] and anterior insula); 2) the left ventromedial prefrontal cortex (BA 10); and 3) the dorsal midbrain. By examining the summed z-score of the functional connectivity of the SCC with these three regions, it was demonstrated that the summed value, when applied to all individual subjects, provides reasonable measures of internal validity (72%−78% for remission; 75%−89% for treatment failure), exceeding the value of any clinical measure. Overall, negative connectivity scores were associated with remission to medication and treatment failure with CBT, whereas positive connectivity scores were associated with remission to CBT and treatment failure with medication. These robust findings indicate that neuroimaging may have an important role in the application of precision medicine for depression by identifying neural signatures of brain states that are differentially responsive to treatments with differing mechanisms of action.
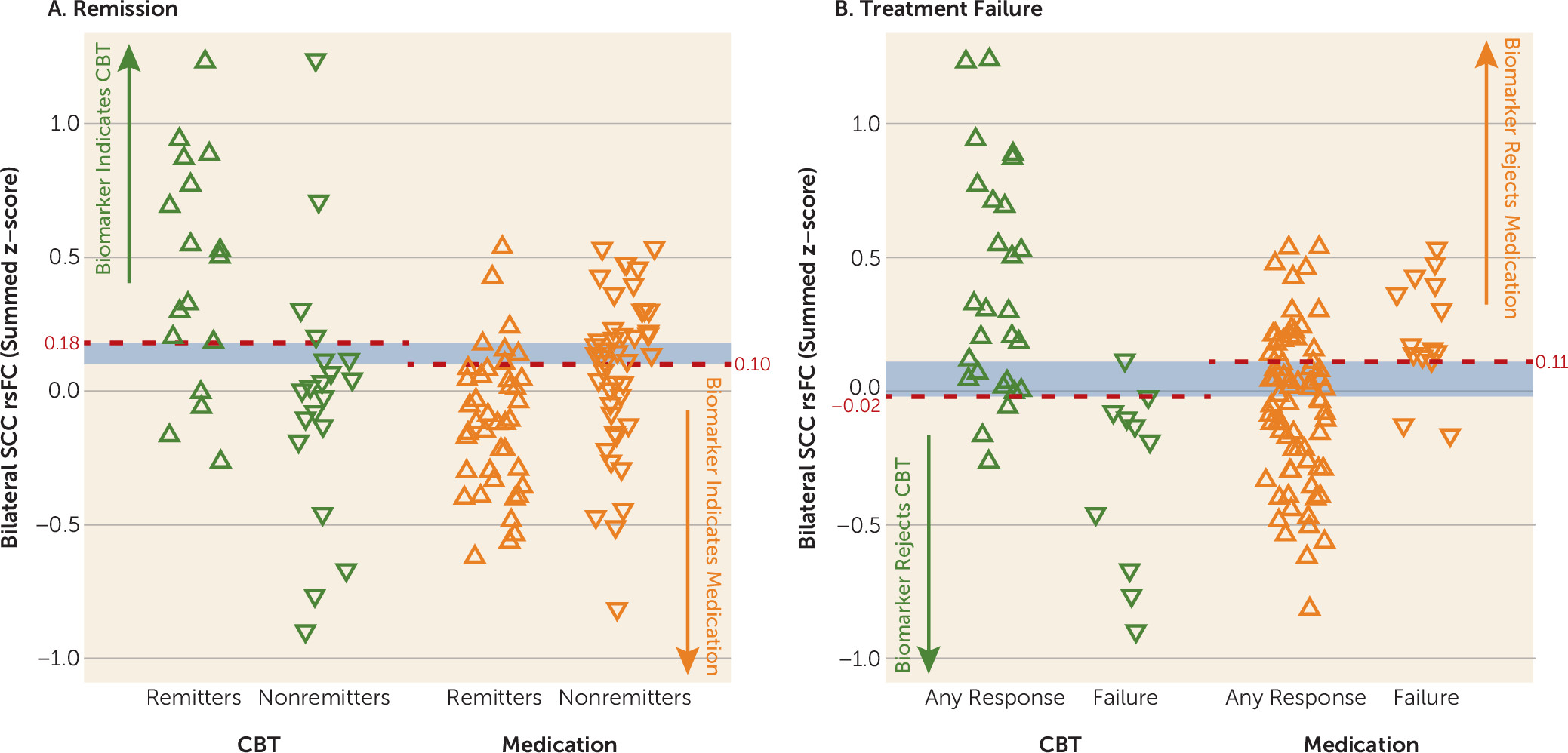
Potential clinical applications of the summed functional connectivity z-score biomarker may depend on the clinical status of the patient and the treatment options available. For patients with profound functional impairment or high suicidality, avoidance of treatment failure may be the treatment priority, whereas for other patients the primary goal may be remission. When remission is the goal, a summed connectivity score >0.18 indicates that CBT should be used, whereas a score <0.10 suggests that medication is indicated. Patients with scores between 0.10 and 0.18 fall into a gray zone, where the biomarker does not suggest a specific treatment (
Figure 3A,
Figure 4A). Alternatively, in situations where avoiding treatment failure is the goal, a summed connectivity score of –0.02 or lower indicates that CBT should not be the initial treatment. Scores >0.11 suggest that medication is not the better choice. Scores between zero and 0.11 do not clearly indicate that one treatment would be superior to the alternative (
Figure 3B,
Figure 4B); treatment selection for these patients may be informed by other markers of likely treatment outcomes. Although these findings are encouraging, attempts at replication of the classification value of these indicators in existing data sets or thorough prospective testing should be undertaken before this imaging-based treatment selection approach is incorporated into routine clinical care.
The current findings are broadly consistent with prior neuroimaging prediction studies in major depression (
15). We have previously proposed that psychotherapy-responsive depression may represent a brain state with sufficiently adequate connectivity in mood-regulating systems such that engagement of these systems via psychotherapy can reduce negative emotional states (
25). Several studies support the conclusion that, on average, patients with major depression have reduced prefrontal control over emotion-generating limbic structures (
29). Greater SCC reactivity (not functional connectivity) to presentations of negative, self-relevant words is associated with poorer outcomes to treatment with CBT (
17). Others have found better response to CBT among major depressive disorder patients who were closest to healthy controls in terms of reactivity to emotional stimuli in the ventromedial prefrontal cortex (
56), VLPFC, dorsolateral prefrontal cortex, and dorsal anterior cingulate cortex (
57).
The VLPFC activity in healthy controls has been linked repeatedly to emotion regulation (
58,
59) and sustained attention (
60). The VLPFC is involved in stimulus selection, and reduced VLPFC activation in response to stimuli is associated with inability to disengage from negative stimuli (
61). Impaired emotion regulation is linked to activity in the VLPFC among patients with major depression (
62), and rumination is associated with VLPFC activity (
63,
64) and volume (
65). Greater resting-state functional connectivity between the SCC and VLPF47 may reflect the availability of this system to be recruited for mood regulation and therefore the ability to respond to CBT.
The operculo-insular cortex (VLPF47/INS region) is important for interoceptive and emotional processing (
66,
67). In our previous report using PET, resting-state metabolic activity in the right anterior insula (with the cluster extending into the frontal operculum) differentially predicted remission and treatment failure with CBT and escitalopram (
27,
28). Although the prior PET study differed from the current study in its methodology and the amount of prior treatment of the evaluated patients, and although the findings differed by side (right versus left), taken together the studies suggest that abnormal metabolic activity in regions associated with interoception and mood regulation may be an important predictor of outcomes to differing forms of treatment. From a clinical perspective, cases of major depression more associated with signals from the body (“gut feelings”) may be more resistant to pure psychotherapy approaches, whereas major depression that does not involve strong interoceptive experiences may be particularly responsive to CBT (
68).
Functional connectivity of frontal brain regions with the midbrain has not emerged in prior fMRI analyses of major depression or its treatment, though an FDG-PET study found that lower pretreatment resting-state metabolism in the midbrain did predict remission to standard antidepressant medications (
69). The coordinates of the midbrain signal in the present analyses indicated potential involvement of the periaqueductal gray, which is involved in coordinated autonomic and behavioral responses to emotional stimuli. Neuroanatomical analyses of periaqueductal gray connection studies in macaques demonstrated that the subcallosal (BA25) and pregenual (BA 32) frontal regions provided the strongest direct input to the dorsolateral column of the periaqueductal gray identified here (
70). Furthermore, serotonin transporter concentrations may be elevated in the periaqueductal gray of major depression patients compared with healthy control subjects (
71). In healthy controls, periaqueductal gray activity can be modulated by placebo-induced expectation of pain relief, and functional connectivity between SCC and periaqueductal gray is increased during a cold pressor task (
72). This ability to regulate the periaqueductal gray may be diminished in the subset of patients with lower periaqueductal gray-SCC functional connectivity.
Sufficient connectivity between the SCC and the midbrain may also reflect the importance of the structural connections between the ventromedial prefrontal cortex and the dorsal raphe (
73). The ventromedial prefrontal cortex (incorporating the SCC) is crucial for regulating an organism’s response to both controllable and uncontrollable stressors, mediated in part by its regulation of dorsal raphe activity in response to stress (
73,
74). Furthermore, in a chronic social defeat model of depression in mice, deep brain stimulation to the ventromedial prefrontal cortex induces neuroplastic changes in the serotonergic neurons in the dorsal raphe (
75). Weak or absent connectivity between the SCC and dorsal raphe may reflect a biological inability of a patient to achieve effortful control over stress responses and thus indicate the need for a direct effect on the serotonergic transporters and autoreceptors of the raphe by antidepressant medication (
76).
Several prior studies have implicated dysregulation in the medial portion of BA 10 in patients with major depression (
77,
78), and the polar components of VMPF10 may be smaller in patients with major depression compared with healthy controls (
79). The ventromedial portion of BA 10 in the prefrontal cortex is an important component of the default mode network (
80) and is extensively connected to the SCC (via the fronto-medial extent of the uncinate fasciculus) (
38), as well as the periaqueductal gray and hypothalamus (
81,
82). Moreover, VMPF10 and VLPF47 are bidirectionally connected by lateral branches of the uncinate fasciculus, disruptions of which are associated with impairments in use of memory to guide decision making and socio-emotional difficulties (
83). Intriguingly, metabolic activity in the anterior insula and periaqueductal gray of rhesus monkeys correlates positively with anxious temperament behaviors in animals exposed to threat (
84). Taken together, the three regions identified in the current analyses are consistent with an interactive network of regions involved in processing and regulating emotional states. Beyond the findings related to the association with treatment outcomes, these results provide further information regarding the pathophysiology of major depression. It would be informative to examine how the functional connectivity patterns associated with treatment outcomes in the present analysis compare with the functional connectivity of the SCC in age- and gender-matched healthy control subjects and in patients with remitted major depression.
An important strength of the PReDICT study is that all patients were treatment-naive. Prior studies have shown that antidepressant medication treatment alters reactivity of the SCC, VLPFC, and insula (
85), as well as the functional connectivity between cortical and limbic regions in major depression patients (
86). These findings indicate the potential for confounding in neuroimaging studies using patients on antidepressant medications at baseline. The PReDICT study’s treatment-naive sample indicates that treatment-related subtypes are not driven by prior treatment exposures.
Studying treatment-naive patients without substantial comorbidity controlled for variables that could have impaired detection of between-group differences. The treatment-naive sample potentially limits generalizability, but our prior work demonstrated that pretreatment anterior insula metabolism was associated with differential treatment outcomes to medication and CBT in a previously treated, predominantly recurrent, sample of depressed patients (
26). Similar to other searches for predictors, another limitation of the present study is that scans were conducted at a single time point and thus reflect only a cross-sectional (“state”) view into depression pathophysiology. Finally, a placebo-control treatment arm could have helped interpretation of the treatment-specific effects of the imaging findings.
The present results, in conjunction with our prior CBT compared with antidepressant medication study using FDG-PET (
26,
27), argue strongly that brain state subtypes of heterogeneous major depressive disorder patients may reflect their biological capacity to benefit differentially from treatments with differing mechanisms of action. Brain-based measures of major depression are proving superior to clinical measures and patient preferences in signifying differential outcomes to depression treatments (
7,
8,
10). Such measures may provide a basis for possible future algorithms for triaging subjects to the appropriate treatment, likely as a component within a multivariate approach to prediction. Further development of treatment selection biomarkers using replication and prospective testing can be expected to contribute meaningfully to the clinical goals of precision medicine approaches for patients with major depressive disorder.