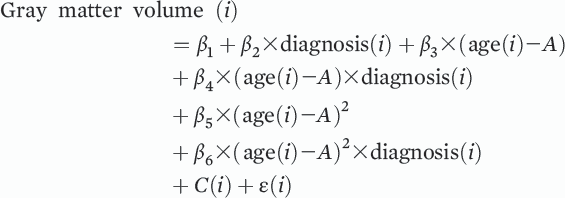Abnormalities in gray matter and white matter structure are consistently observed in schizophrenia and are known to progress over the course of the illness, particularly during the earliest stages of the disorder (
1–
3). In parallel, it is recognized that dynamic changes in brain structure occur with normal brain development, maturation, and aging (
4–
6). Despite consistent observations of reduced gray matter volume and white matter integrity in schizophrenia, it remains unclear whether these deficits become exaggerated with increases in age or duration of illness, or whether the rate of deterioration is constant across the lifespan compared with normal age-related trajectories.
Previous neuroimaging studies have provided some evidence for abnormal brain aging in schizophrenia, as demonstrated by cross-sectional studies examining individuals at different stages of adult life. Supporting propositions that schizophrenia is a syndrome of “accelerated aging” (
7), a number of studies have found that individuals with schizophrenia exhibit an elevated age-related decline in gray matter volume (
8–
10), white matter volume (
11), and fractional anisotropy (
12–
15), where the latter is a composite measure of axonal packing density, diameter, and myelination. However, discordance between these studies is evident, with reports indicating a diminished rate (
11,
16,
17) or no difference (
18) in the rate of age-related brain structural loss in the illness. More recently, machine learning has been used to predict the age of an individual based on gray matter density maps derived from structural MRI (
19,
20). When applied to patients with schizophrenia, the predicted age, or the so-called brain age, was, on average, more than 3 years greater than a patient’s chronological age, with this apparent age gap increasing at follow-up. Interestingly, the rate of brain aging was rapid at illness onset and plateaued 5 years after onset (
20). Together, these studies suggest that an exaggerated age-related decline of gray matter and white matter manifests in schizophrenia, particularly at the earliest stages of the disorder.
Although such studies are informative, several questions concerning the nature, extent, and timing of brain structural changes with aging in schizophrenia remain. First, it is unclear whether age-related gray matter and white matter pathology in schizophrenia follows the same or different trajectories across the lifespan. To date, no studies have simultaneously examined both gray matter and white matter with age in schizophrenia; thus, it is not clear if gray matter changes precede white matter changes or vice versa. Second, the majority of published studies have consisted of small to medium sample sizes (<70 individuals per group). This may explain some of the heterogeneity in findings, due to insufficient power to model the influence of age, particularly if the age of a cohort is distributed within a narrow range. Third, most studies have examined the influence of age on global (total or whole-brain averaged) brain measures or on a restricted number of regions, thus precluding the examination of age-related effects across brain regions or outside the specific regions sampled. Given that different brain regions, such as the prefrontal cortices, are more vulnerable to aging than others (
4), it is possible that individuals with schizophrenia show exaggerated age-related trajectories only in specific brain areas. Few previous studies have modeled the effect of age in schizophrenia on a voxel-by-voxel basis, unrestricted by a priori selection of regions (
9,
18). Finally, the majority of previous studies have employed linear modeling of age-related effects, despite evidence of nonlinear trajectories of brain structural change with age both in individuals with schizophrenia and in healthy individuals (
5,
10).
Using structural and diffusion-weighted imaging of brain anatomy in an age-diverse cross-sectional design, the present study examined change in gray matter and white matter over the lifespan in a large sample of schizophrenia patients and healthy subjects. We aimed to determine whether the rate of change of structural neuropathology associated with schizophrenia was stable, accelerated, or diminished with age. We also aimed to determine whether any periods showing an altered rate of change coincided between gray matter and white matter or followed largely independent trajectories. We hypothesized that schizophrenia patients would show accelerated gray matter and white matter trajectories with age, particularly in early adulthood, corresponding to the early stages of illness.
Discussion
This cross-sectional imaging study examined age-related changes in gray matter volume and fractional anisotropy in a large cohort of patients with established schizophrenia and healthy comparison subjects. We aimed to determine the pattern of gray matter and white matter trajectories to assess whether the structural neuropathology associated with schizophrenia was stable, accelerated, or diminished with age.
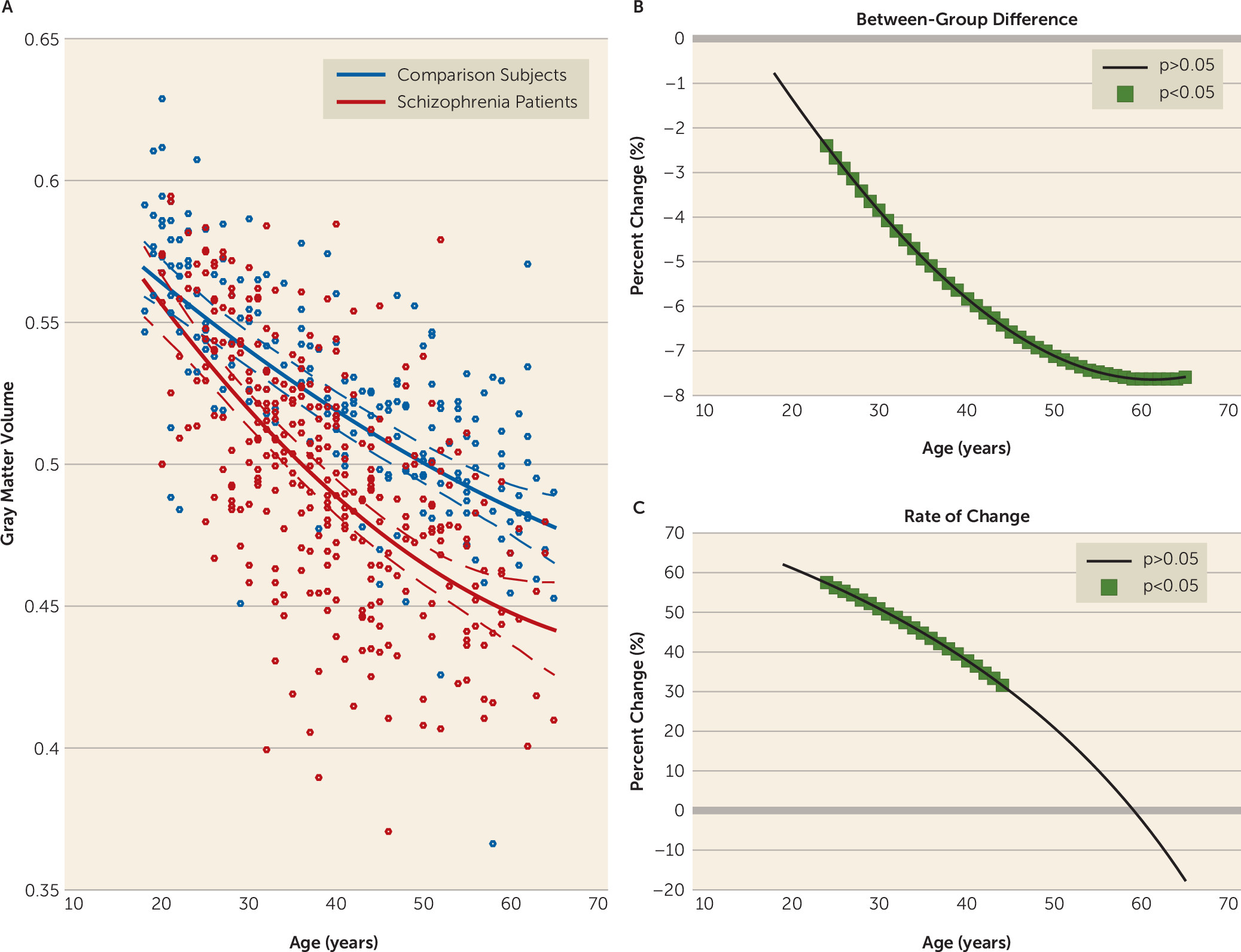
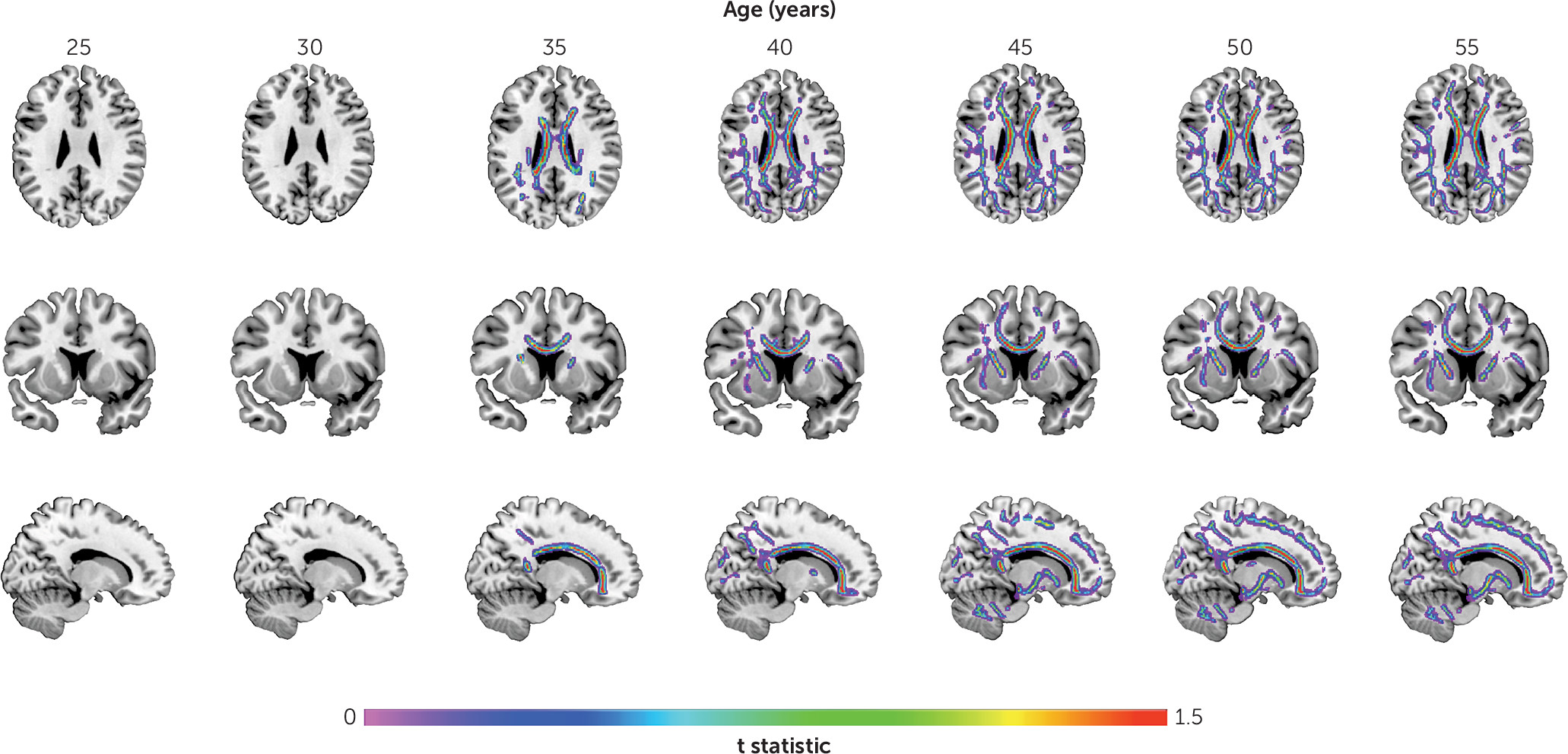
We found larger age-related abnormalities of both gray matter volume and fractional anisotropy in schizophrenia compared with comparison subjects. Interestingly, we also found that the time course of these structural pathologies differed. Specifically, we found that the loss of gray matter volume in schizophrenia was evident from young adulthood, with the rate of loss being significantly faster in schizophrenia until middle age, slowing thereafter to a stable rate. In contrast, the rate of age-related white matter decline was constant across the lifespan and best modeled as a linear function. The rate of white matter decline was 60% steeper in schizophrenia compared with comparison subjects, with significant between-group differences emerging in midlife and progressively widening between the two groups with age. These findings suggest that schizophrenia is characterized by early loss of gray matter followed by white matter deficits that widen with age at a constant rate. Importantly, while males showed more severe deficits with age, this pattern of differences by sex did not differ between patients and comparison subjects (see the
online data supplement), a finding consistent with other studies (
28).
Our findings are consistent with previous studies demonstrating elevated age-related decline in gray (
8–
10) and white (
11–
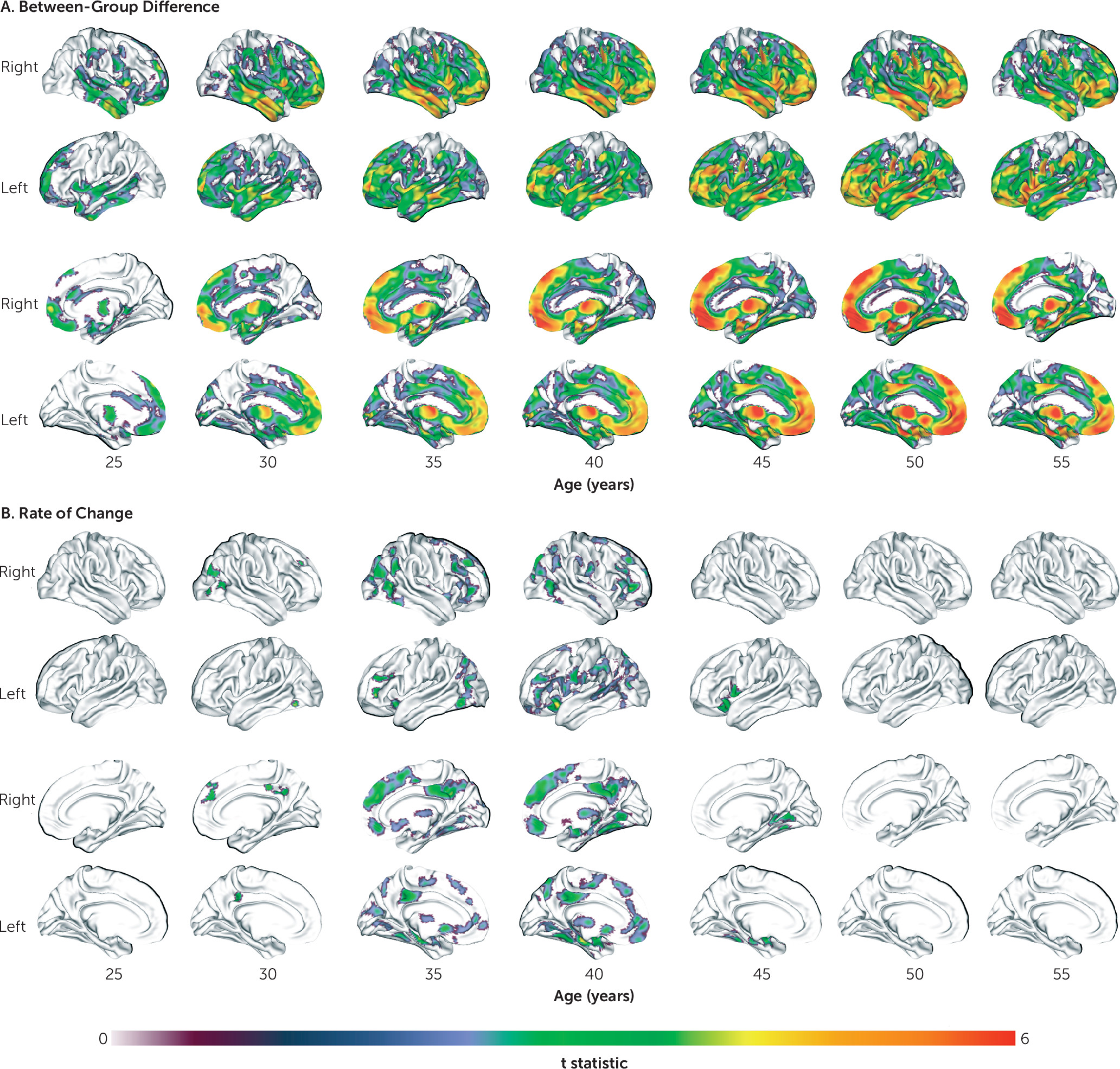
14) matter in schizophrenia. However, we extend these findings by characterizing the regional pattern, nature, and timing of these age-related trajectories. Although gray matter volume was reduced in schizophrenia at all ages, this reduction was initially confined to frontotemporal regions in young adulthood before becoming more widespread by middle age. Frontotemporal volume loss is consistently reported in schizophrenia across a range of illness and maturational stages (
29–
31). Because, in our sample, this circumscribed loss coincides with the end of the maturational posterior-to-anterior gradient of gray matter loss (
32), this reduction may reflect accelerated neuromaturational processes. Interestingly, the regional pattern of gray matter volume loss in schizophrenia followed a largely anterior-to-posterior direction with increasing age, targeting frontal and temporal regions first before extending to parietal and then occipital and cerebellar areas, while sensorimotor areas remained relatively preserved. This pattern supports the “last-in, first-out” hypothesis of brain aging, whereby late-developing regions are thought to degenerate first (
33), and it suggests that this process might be exaggerated in schizophrenia.
In addition to gray matter volume being reduced in schizophrenia at all studied ages, we found that it declined significantly faster in patients within a discrete age window, specifically between early adulthood and middle age, with the steepest rate found between 20 and 30 years of age. Because our patients in this age band had a diagnosis of schizophrenia for an average of 6.5 years, these findings are consistent with longitudinal studies showing that the most pronounced cortical volume loss occurs in the early stages of the illness (
2,
20). However, unlike these studies, ours found that a faster rate of decline of gray matter volume continued into middle life, which corresponded to around 15 years of illness in our sample. Consistent with other studies (
34), this finding suggests that individuals with schizophrenia continue to lose gray matter volume beyond the first years of illness—up to 15 years postillness—albeit at a relatively slower rate. Regionally, this faster age-related loss was more widespread than previously reported (
9,
10) and affected a number of cortical and subcortical regions within both hemispheres. By age 50, the rate of gray matter volume loss in schizophrenia had stabilized to rates seen in healthy comparison subjects and was actually more than 10% slower in schizophrenia after age 60, although this slowing was not significant. It remains to be investigated whether a decelerated rate of gray matter volume loss in schizophrenia continues into senescence. Our gray matter volume results suggest that there is a period of accelerated gray matter volume decline occurring against a background of extensive anterior-to-posterior gray matter volume loss with age in individuals with established schizophrenia, with the pattern of gray matter volume change possibly reflecting a disruption of both late maturational (e.g., synaptic pruning [
35]) and early aging processes.
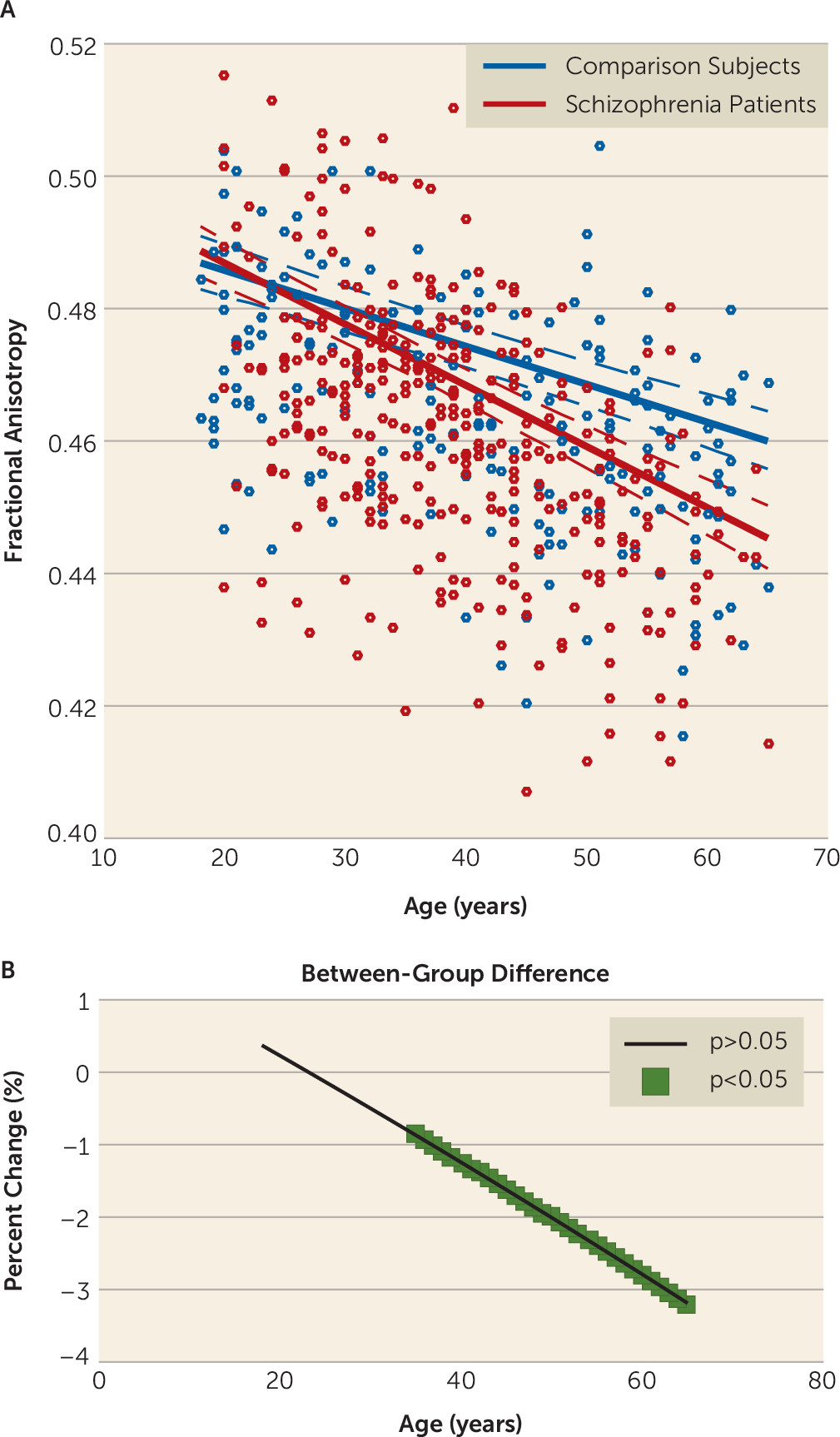
In contrast to gray matter volume, fractional anisotropy was unimpaired in individuals with schizophrenia at earlier ages. Although most studies of young people with schizophrenia have demonstrated reduced fractional anisotropy relative to comparison subjects, heterogeneity still exists with reports of increases and of no changes in fractional anisotropy (
36,
37), possibly owing to variability in maturational and illness stages, clinical profile, duration of symptoms, and level of acuity, as well as sample size and regions selected (
37). While deficits in fractional anisotropy have been observed in first-episode patients, our young patients had established schizophrenia of variable duration and were relatively stabilized; thus, illness-specific deficits relevant to first presentation of illness may not have been identified. Despite fractional anisotropy being unchanged in patients in early adulthood, significant reductions were present from midlife, and these reductions followed a linear decline, becoming widespread and more marked with increasing age. This finding replicates studies demonstrating a linear decline of fractional anisotropy with age in healthy subjects (
6) and an elevated age-related decline in schizophrenia (
12–
14). However, like those found for gray matter volume, the fractional anisotropy deficits were more spatially extensive, possibly because our large sample size allowed us to detect subtle effects. Inconsistent findings have been reported, however, with some studies indicating that fractional anisotropy deficits in schizophrenia recover with age (
16,
17).
A novel finding of this study is that neurostructural age-related abnormalities in schizophrenia follow a different temporal sequence in gray matter and white matter. Specifically, increased white matter pathology lagged that seen for gray matter, suggesting that gray matter changes precede white matter change in adults with schizophrenia. One possibility for this temporal pattern is that white matter deficits are an epiphenomenon of gray matter deficits such that they are secondary to, or a consequence of, progressive gray matter loss. Another possibility is that both deficits are a consequence of neurodevelopmental and aging processes that manifest differently throughout the disease (
38). Interestingly, we found that fractional anisotropy deficits in schizophrenia lagged those seen in gray matter volume by about a decade, a result that parallels the timing of peak maturation in gray matter and white matter, whereby cerebral white matter matures about a decade after gray matter (
39). Although the precise mechanisms underlying the accelerated age-related changes in gray matter and white matter are unclear, a number of shared or independent factors could contribute, including genetic background (
40), inflammation (
41), and lifestyle factors such as low socioeconomic status, poor nutrition, physical illness, inactivity, and substance use (
42,
43), all of which are reported to be high in this population (
44).
The main strengths of this study were the large sample (>500 subjects), the neurostructural mapping of both gray matter and white matter, and the age-specific localization of the neurostructural effects. In addition to these strengths, several limitations should be noted. First, our study was limited by the use of a cross-sectional design to infer age-related changes in brain structure. Therefore, cohort effects (generational and illness-related) may have confounded our findings. Although trajectories of brain aging would be best examined with a longitudinal design, performing such studies over the lifespan is largely infeasible. Therefore, the disadvantages of a cross-sectional design must be considered against the advantages of our very large sample size spanning the adult lifespan for both gray matter and white matter. Second, our age range of 20–65 years precluded examination of brain trajectories related to early development and old age degeneration, which may be altered in schizophrenia differently in gray matter and white matter. Third, as in most studies of schizophrenia, we were unable to comprehensively examine the influence of medication given the unavailability of dosage and history information. Nevertheless, we found no evidence of a difference in trajectories of gray and white matter aging between patients not currently treated with antipsychotics compared with those treated with typical or atypical antipsychotics. Interestingly, antidepressant treatment was associated with higher fractional anisotropy, suggesting a possible neuroprotective action of the drug (
45). Alternatively, patients medicated with antidepressants might represent a specific clinical subgroup characterized by improved white matter integrity. Although antidepressant use was significantly associated with a diagnosis of schizoaffective disorder, we found no group differences in fractional anisotropy between schizophrenia and schizoaffective disorder. Furthermore, patients treated and not treated with antidepressants did not differ on negative and positive symptoms, suggesting that patients treated with antidepressants do not represent a better clinical prognosis, at least symptomatically. Further studies are needed to clarify the association between antidepressant use and white matter integrity in schizophrenia. Finally, as age and duration of illness were positively correlated, and as the duration of illness was correlated with gray matter volume and fractional anisotropy, our findings in schizophrenia can also be interpreted in terms of illness duration because age and illness duration are inextricably linked.
In conclusion, we identified significant differences in gray matter volume and white matter integrity as a function of age in individuals with schizophrenia relative to healthy comparison subjects. We demonstrated faster age-related gray matter and white matter decline in schizophrenia at distinct age windows, with accelerated gray matter loss evident in early to mid-adulthood, while white matter deficits lagged gray matter loss, widening with age at a constant rate.