Prospective serial depression treatment trials demonstrate that more than 60% of patients with major depressive disorder fail to remit with an initial pharmacotherapy trial, and a progressively smaller proportion of patients remit with each subsequent trial, until the remission rate after a fourth antidepressant trial is between 10% and 15% (
1–
4). Treatment-resistant depression refers to major depression that fails to remit after at least two separate and adequate trials of antidepressants from two different pharmacological classes. The Sequenced Treatment Alternatives to Relieve Depression trial (which did not include patients with bipolar disorders) showed that 32% to 41% of patients with treatment-resistant depression fail to remit after four trials of antidepressants, resulting in a large population of symptomatically and functionally impaired individuals (
1,
5).
Vagus nerve stimulation (VNS) has been shown to be efficacious for the long-term management of patients with treatment-resistant depression (
6,
7) and is approved by the U.S. Food and Drug Administration (FDA) as an adjunctive treatment for treatment-resistant depression. APA recommends VNS as a treatment option for patients who have not responded to at least four adequate trials of depression treatments, including ECT (
8).
As a condition for approval of the treatment-resistant depression indication for VNS, the FDA required a postmarketing surveillance study, and therefore the Treatment-Resistant Depression Registry was established in 2006 as a long-term, prospective, multicenter, open-label, nonrandomized, longitudinal, naturalistic, observational study to follow the clinical course and outcome over 5 years in two large cohorts of patients with treatment-resistant depression. Registry patients received either treatment as usual only (the treatment-as-usual arm) or treatment as usual with adjunctive VNS (the VNS arm), and the FDA agreed on a planned enrollment of 500 patients in the VNS arm and 300 patients in the treatment-as-usual arm. We hypothesized that the VNS arm would have superior clinical outcomes, based on long-term depression and mortality, compared with the treatment-as-usual arm. Here we report the 5-year findings from this registry, comparing treatment outcomes in the two groups, including response, remission, suicidality, and mortality, along with subanalyses of patients with a history of response or nonresponse to ECT, patients with comorbid generalized anxiety disorder, and patients with bipolar versus unipolar depression.
Method
The Treatment-Resistant Depression Registry
The registry included 61 sites in the United States representing academic, institutional, and private clinic settings that specialized in treatment of depression. The registry was approved by an institutional review board, and written informed consent was obtained from all study patients after the procedures had been fully explained. Registry data were collected between January 2006 and May 2015.
Patients were recruited by physician referral from all participating sites. They included patients who were being evaluated for surgery or anesthesia to undergo VNS implantation, patients who had signed surgical or anesthesia consent forms to receive a VNS device, patients who had a scheduled VNS implantation surgery, and patients who had completed participation in the VNS dose-finding study, referred to as the D-21 study (
ClinicalTrials.gov identifier: NCT00305565).
Based on a study design in agreement with the FDA, the VNS arm in the registry included “new patients” (335 patients without prior VNS treatment) and “D-21 rollover patients” (159 patients who received VNS treatment in the D-21 study, which was being conducted simultaneously with the registry study; these patients rolled over into the registry after completing participation in D-21 as they fulfilled the registry’s entry criteria) (
9). Sensitivity analyses showed that there were no differences between the VNS arm (with or without the D-21 rollover patients) and the treatment-as-usual arm for any of the efficacy assessments (see the Supplemental Methods section of the
data supplement that accompanies the online edition of this article). Therefore, in agreement with the FDA, the VNS pooled data were included in the registry data analyses.
For the D-21 rollover patients, the Clinical Global Impressions severity (CGI-S) score (
10) from the D-21 screening visit was used to assess eligibility for enrollment in the registry. Data prior to VNS device implantation (baseline) and up to 1 year after implantation were included in the registry data set. Based on the time lapse from the original D-21 implantation surgery, D-21 rollover patients entered at the corresponding follow-up time point in the registry.
To be eligible for enrollment in the registry, patients had to be age 18 or older; have a current major depressive episode (according to DSM-IV-TR criteria and confirmed by the Mini International Neuropsychiatric Interview) (
11) of ≥2 years in duration (unipolar or bipolar depression) or have a history of at least three depressive episodes including the current major depressive episode; and have a history of inadequate response to at least four depression treatments (including maintenance pharmacotherapy, defined as dosage per Physician’s Desk Reference labeling for a minimum of 4 weeks, psychotherapy, and ECT). Diagnoses of psychiatric conditions were made by trained psychiatrists at each recruiting site. The following additional entry criteria were applicable prior to enrolling in either the registry or the D-21 study: a CGI-S score ≥4; no history of schizophrenia, schizoaffective disorder, any other psychotic disorder, or a current major depressive episode that included psychotic features; not currently psychotic; no history of rapid-cycling bipolar disorder; and no previous use of VNS (other than the D-21 rollover patients).
Before designating any enrolled patient as being lost to follow-up, the study site made at least two attempts to contact the patient (by either telephone or certified mail) and encouraged the patient to complete the exit form and perform a final follow-up visit.
Study Treatment and Outcome Measures
Prior to enrollment, registry patients (except for the D-21 rollover patients) were allowed to select the treatment arm of their choice; however, patients could also be assigned by the site to receive the alternate treatment for various reasons, including availability of surgical implantation at a site, number of allocated slots for implantation, availability of VNS devices that had been donated by the registry sponsor, or failure to qualify for insurance reimbursement for VNS implantation. The costs associated with device implantation surgery and related medical care during registry participation were covered by either the patient or the patient’s health insurance policy.
Patients in the VNS arm underwent the implantation surgery before visit 2 (baseline). Postbaseline follow-up visits for all patients were scheduled to occur at 3, 6, 9, 12, 18, 24, 30, 36, 42, 48, 54, and 60 months, at which data were collected on medical status, adjustment of mood disorder therapy (as needed in the judgment of the clinician), and concomitant treatments (there were no restrictions on concomitant treatments in this observational registry). The Montgomery-Åsberg Depression Rating Scale (MADRS) (
12) (administered by central raters), the Quick Inventory of Depressive Symptomatology–Self Report (QIDS-SR) (
13,
14), the CGI improvement (CGI-I) score, and the patient-rated Frequency, Intensity, and Burden of Side Effects Rating scale (
15) were administered, and data were collected on mortality and suicidality (
16).
After each patient visit, the site notified the central raters to initiate patient follow-up via telephone, which was conducted separately from the site visit. Central raters were unblinded nurses trained to assess suicidality. The central raters maintained continuous progress notes on each patient throughout the study and alerted investigators if any suicidal thoughts or actions had occurred or, in their opinion, might occur.
Suicidality was assessed on the basis of three measures: a score of 2 or 3 on QIDS-SR item 12 (corresponding to the responses “think of suicide or death several times a week for several minutes” to “have actually tried to take my life”), a response of “yes” to the question “Has the patient made a suicidal gesture or attempt since the last visit?” on the investigator-completed suicidality assessment, and a score ≥4 on MADRS item 10 (corresponding to the responses “probably better off dead” and “active preparations for suicide”).
Analysis Populations and Statistical Analysis
The safety analysis population included patients in the treatment-as-usual arm who had completed the visit 2 (baseline) requirements and patients in the VNS arm who had undergone VNS device implantation before visit 2. The intent-to-treat population included patients who completed their baseline visit, received their respective treatment, and completed at least one postbaseline assessment.
All statistical analyses were performed using SAS, version 9.3 (SAS Institute, Cary, N.C.). For inferential statistics and tests of hypotheses, analysis of variance models using PROC GLM in SAS were used for continuous variables for visit-wise comparisons of treatments. For modeling of nonlongitudinal categorical data, logistic regression models were used (PROC LOGISTIC) in SAS. The mixed-model repeated-measure models utilizing SAS PROC MIXED and/or generalized linear mixed model (PROC GLIMMIX) were fitted for longitudinal data involving repeated measures over time. Time-to-event analyses were summarized using Kaplan-Meier curves and Cox regression models. Log-rank testing was used to test the null hypothesis of equal event time distributions between treatment arms. Summary descriptive statistics were generated for continuous variables, and frequencies and percentages for categorical variables. Tests were two-sided, with a type I error rate of 5% at the 95% confidence level.
The propensity score method (
17) was used to adjust for imbalance of baseline prognostic factors between treatment arms. The patient propensity scores derived from the modeling were stratified into quintiles, and the stratified quintiles variable was incorporated as a classification variable in the final models and hypothesis testing of treatment differences.
The primary efficacy endpoint was the percentage of responders in each treatment arm through 5 years of follow-up, with response defined as a reduction of ≥50% from baseline MADRS score at any postbaseline visit. Secondary efficacy endpoints included response based on a CGI-I score of 1 or 2 at a postbaseline visit and an improvement of ≥50% from baseline on the QIDS-SR; remission, based on a MADRS score ≤9 at a postbaseline visit, a QIDS-SR score ≤5 at a postbaseline visit, and a CGI-I score of 1 at a postbaseline visit; and duration of remission, based on time from first remission (MADRS score ≤9) to first recurrence (MADRS score ≥20). Safety endpoints, specifically suicidality, were analyzed using PROC GLIMMIX based on whether the risk was greater or less than it was at baseline, or unchanged.
There were no imputations of missing data. The use of mixed-model repeated-measure modeling was preferred because the application is simple and produces results similar to those of multiple imputations, and with the same assumptions (
18,
19). Additional sensitivity analysis checks were conducted, including percentage of missing data that were found to be similar between the two treatment arms; a non-missing visit-wise analysis that showed the same trend as the primary mixed-model repeated-measure modeling results; and a tipping-point analysis to rule out missing-not-at-random bias, as opposed to missing at random (
20,
21), which confirmed that there were no realistic deviations from missing-at-random assumptions, thus confirming the appropriateness of the primary analysis.
Results
The safety analysis population included 795 patients—494 patients in the VNS arm (including 159 D-21 rollover patients) and 301 patients in the treatment-as-usual arm (see the Supplemental Results section of the
online data supplement). A diagnosis of severe recurrent major depressive disorder was reported in 46% of patients in the VNS arm and 32% of patients in the treatment-as-usual arm (
Table 1). About 27% (N=134) of patients in the VNS arm and about 24% (N=71) of patients in the treatment-as-usual arm had a primary diagnosis of bipolar I or bipolar II disorder. Other diagnoses and their frequencies are listed in
Table 1.
At baseline, the mean number of failed treatments for depression was 8.2 (SD=3.3) in the VNS arm and 7.3 (SD=2.9) in the treatment-as-usual arm, and the mean lifetime number of attempted suicides was 1.8 (SD=4.0) in the VNS arm and 1.2 (SD=2.4) in the treatment-as-usual arm. The mean baseline MADRS scores for the two groups indicated moderate to severe depression. Overall, the patients enrolled in the VNS arm were more likely to have had ECT exposure, psychiatric hospitalizations, and suicide attempts, and they had higher mean depression rating scale scores, suggesting that they had more severe illness than those in the treatment-as-usual arm (
Table 1).
Of the 494 patients in the VNS arm, 461 (93%), 289 (59%), 313 (63%), 334 (68%), and 300 (61%), respectively, completed years 1, 2, 3, 4, and 5 of the registry (the variable numbers in the VNS arm are due to D-21 patients who rolled over into the registry at various time points after implantation). Of the 301 patients in treatment-as-usual arm, 224 (74%), 185 (62%), 168 (56%), 149 (50%), and 138 (46%), respectively, completed years 1, 2, 3, 4, and 5 of the registry. Of the 358 patients (45%) who withdrew early, 195 were from the VNS arm (40%) and 163 were from the treatment-as-usual arm (54%). The reasons for early withdrawal were similar between the treatment arms (
Table 1).
A total of 765 patients (489 in the VNS arm and 276 in the treatment-as-usual arm) met the criteria for the intent-to-treat population and were included in the efficacy analyses.
Patients could switch to the alternate treatment arm, and 22 patients elected to do so during the study; however, per protocol, data collected after a patient switched treatment arm were censored from the efficacy analysis.
Primary Efficacy Evaluation of Response
A statistically significant difference was observed in the response rate between the VNS arm and the treatment-as-usual arm through the 5-year follow-up period (cumulative response rates, 67.6% [95% CI=63.4, 71.7] and 40.9% [95% CI=35.4, 47.1], respectively; p<0.001).
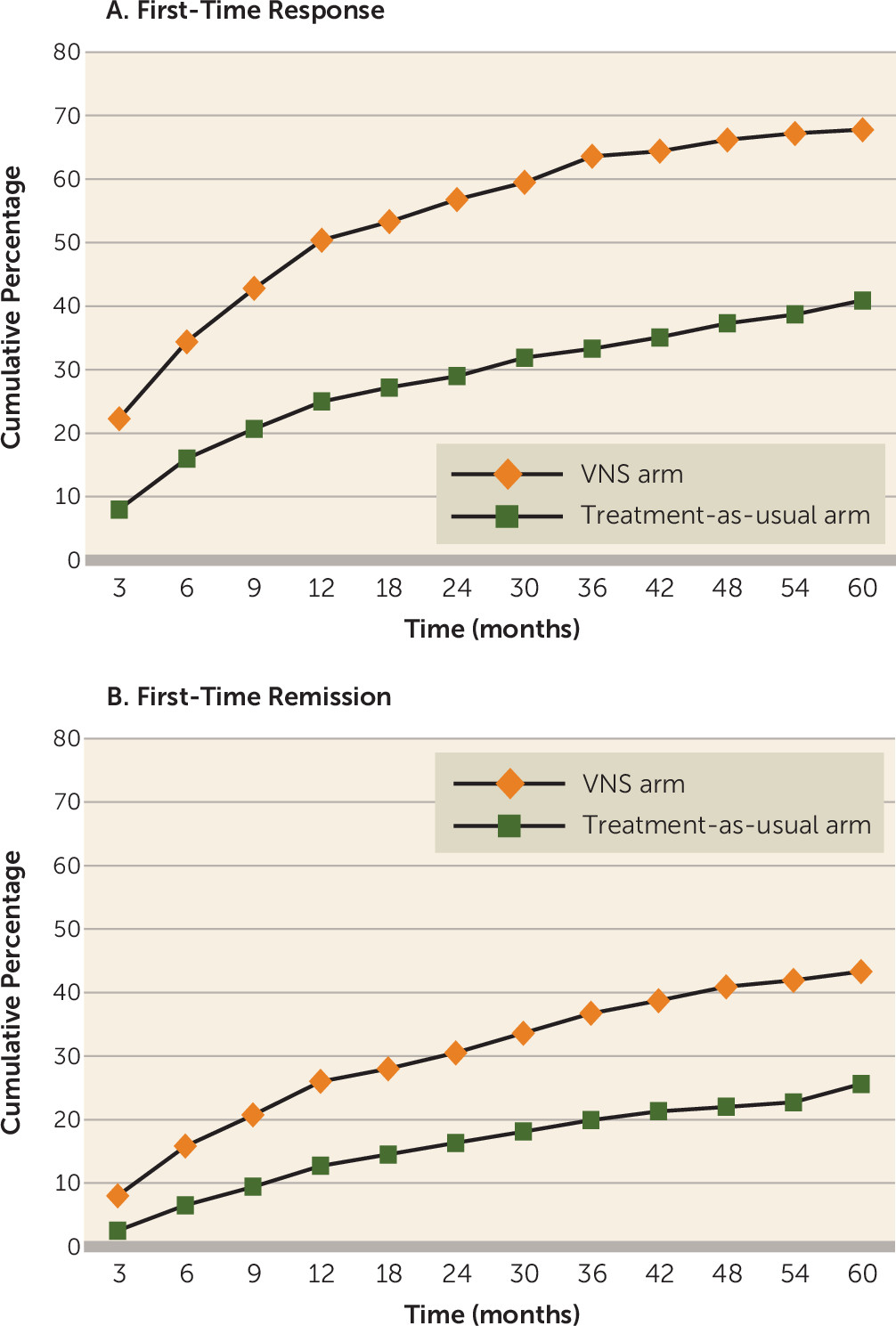
Figure 1A presents the cumulative percentage of first-time responders through the 5-year follow-up period, based on MADRS score. The cumulative percentage of first-time responders in the VNS arm was approximately double that in the treatment-as-usual arm at all postbaseline time points.
Secondary Efficacy Measures
Response based on CGI-I and QIDS-SR.
The proportion of responders for the 5-year follow-up data was also evaluated using CGI-I score (cumulative response rate, 75.9% [95% CI=72.3, 79.9] in the VNS arm and 48.6% [95% CI=43.0, 54.8] in the treatment-as-usual arm; p<0.001) and QIDS-SR score (cumulative response rate of, 64.7% [95% CI=60.7, 69.2] in the VNS arm and 41.7% [95% CI=35.9, 47.5] in the treatment-as-usual arm; p<0.001), and the results were consistent with the findings based on MADRS scores.
Remission.
Figure 1B presents the cumulative percentage of first-time remitters through the 5-year follow-up period, based on MADRS score. Analysis of cumulative remission (based on a MADRS total score ≤9 at any postbaseline visit) demonstrated that over time, patients in the VNS arm were significantly more likely to experience remission than those in the treatment-as-usual arm (43.3% [95% CI=38.9, 47.7] and 25.7% [95% CI=20.7, 31.1], respectively; p<0.001). Based on QIDS-SR scores, there was a statistically significant difference in remission between the VNS and treatment-as-usual arms (40.4% [95% CI=36.2, 44.9] and 25.0% [95% CI=19.9, 30.1], respectively; p<0.001). Likewise, based on CGI-I scores, there was a statistically significant difference in remission between the VNS and treatment-as-usual arms (49.7% [95% CI=45.5, 54.3] and 21.4% [95% CI=16.7, 26.4], respectively; p<0.001).
Time to first response and duration of response.
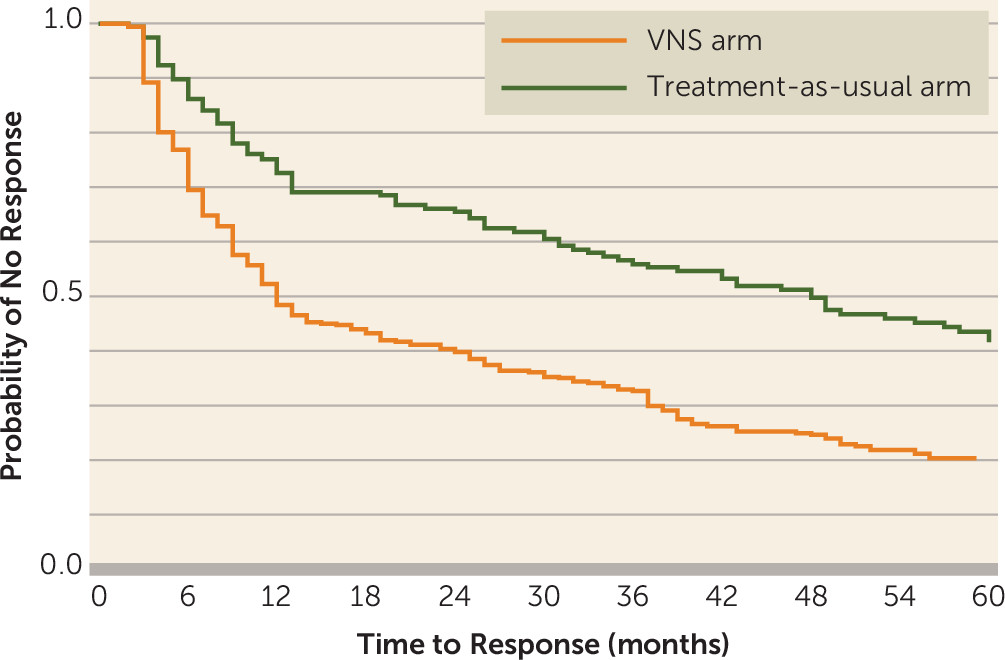
Figure 2 presents the Kaplan-Meier graph of time to first response for each treatment arm, based on MADRS scores. Median time to first response was significantly shorter for patients in the VNS arm than for those in the treatment-as-usual arm (12 months compared with 48 months; p<0.001).
Response duration was assessed by fitting Kaplan-Meier curves of MADRS scores for each treatment arm. Patients in the VNS arm had a significantly longer median time to recurrence than patients in the treatment-as-usual arm (12 months compared with 7 months; p=0.001) (data not shown).
Time to first response and duration of response were also evaluated based on QIDS-SR scores. Median time to first response was significantly shorter for patients in the VNS arm than for those in the treatment-as-usual arm (22 months compared with 47 months; p<0.001). In addition, responders in the VNS arm had a longer median time to recurrence than did responders in the treatment-as-usual arm (10 months compared with 7 months), but this difference did not reach statistical significance (p=0.14).
Time to first remission and duration of remission.
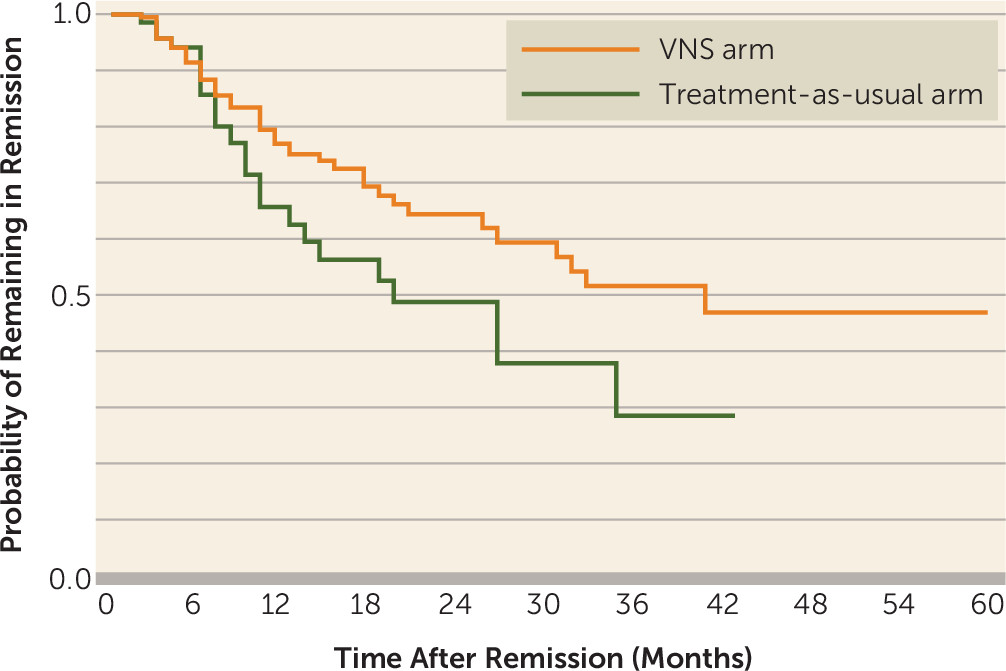
Summary analysis of the Kaplan-Meier time to first remission based on the MADRS data demonstrated that patients in the VNS arm had a significantly shorter median time to remission than patients in the treatment-as-usual arm (49 months compared with 65 months; p<0.001).
Figure 3 presents the median duration of remission (for those patients who remitted) with time to recurrence. The duration of remission based on the MADRS data was longer for patients in the VNS arm than for those in the treatment-as-usual arm (40 months compared with 19 months), but the difference did not reach statistical significance (p=0.10). Similarly, the duration of remission based on the QIDS-SR data was longer for patients in the VNS arm than for those in the treatment-as-usual arm (30 months compared with 18 months), but the difference did not reach statistical significance (p=0.20).
Subanalysis Based on Prior ECT Exposure, Comorbid Anxiety, and Bipolar Depression
A subanalysis was performed in registry patients who had previously completed one or more adequate courses of ECT (defined as at least seven right unilateral treatments). This subanalysis, which included 290 patients in the VNS arm (58.7%) and 109 patients in the treatment-as-usual arm (36.2%), compared cumulative response rates after grouping the patients by their history of response to ECT based on a review of medical records.
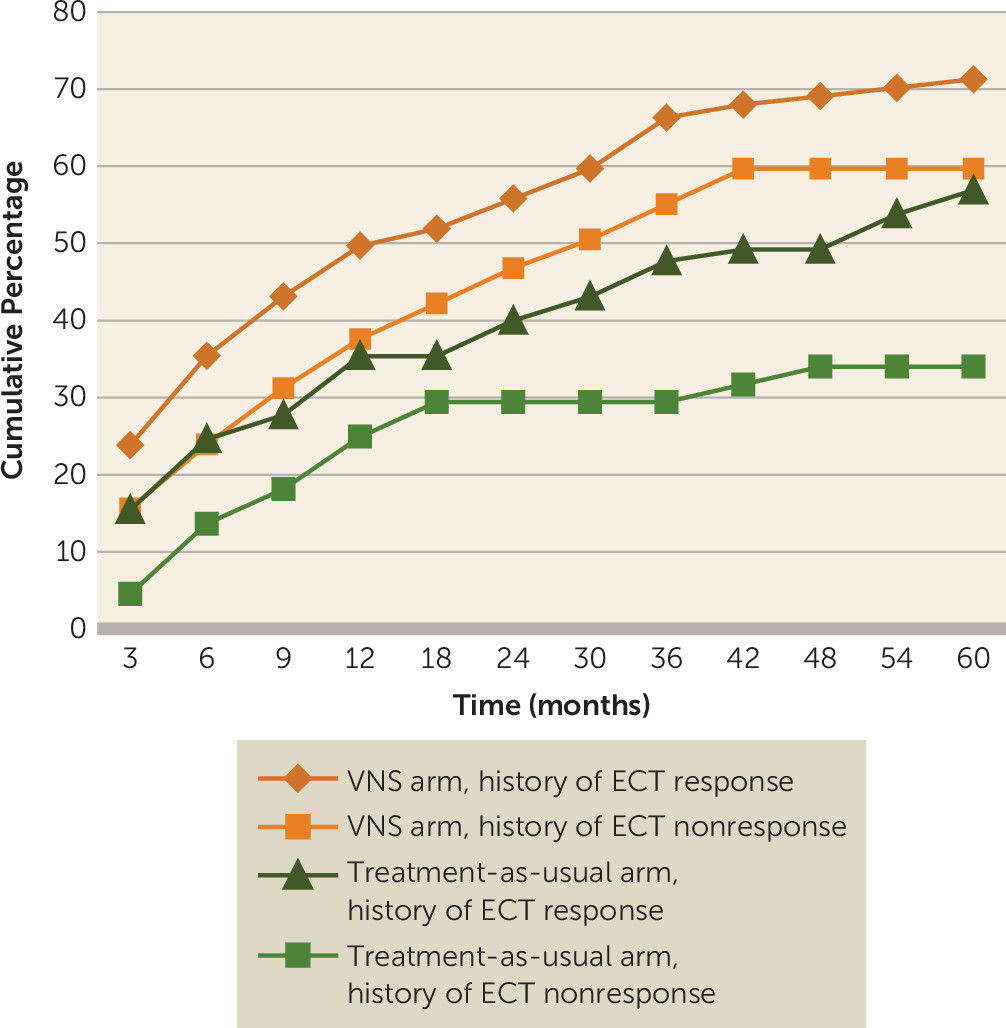
For patients included in this subanalysis, the cumulative percentage of first-time responders through the 5-year follow-up period based on MADRS score is presented in
Figure 4. The 5-year cumulative response rate for patients in the VNS arm who had previously responded to ECT was 71.3% (95% CI=64.3, 77.4), compared with 56.9% (95% CI=44.8, 68.2) for the ECT responders in the treatment-as-usual arm, a statistically significant difference (p=0.006). In addition, a significant difference in response was seen at 9 months, and it was maintained for the duration of the study. For ECT nonresponders in the VNS arm, the response rate was 59.6% (95% CI=50.2, 68.4), compared with 34.1% (95% CI=21.8, 48.9) for ECT nonresponders in the treatment-as-usual arm (p<0.001), with statistically significant separation beginning after 2 years of treatment and continuing until completion of registry participation.
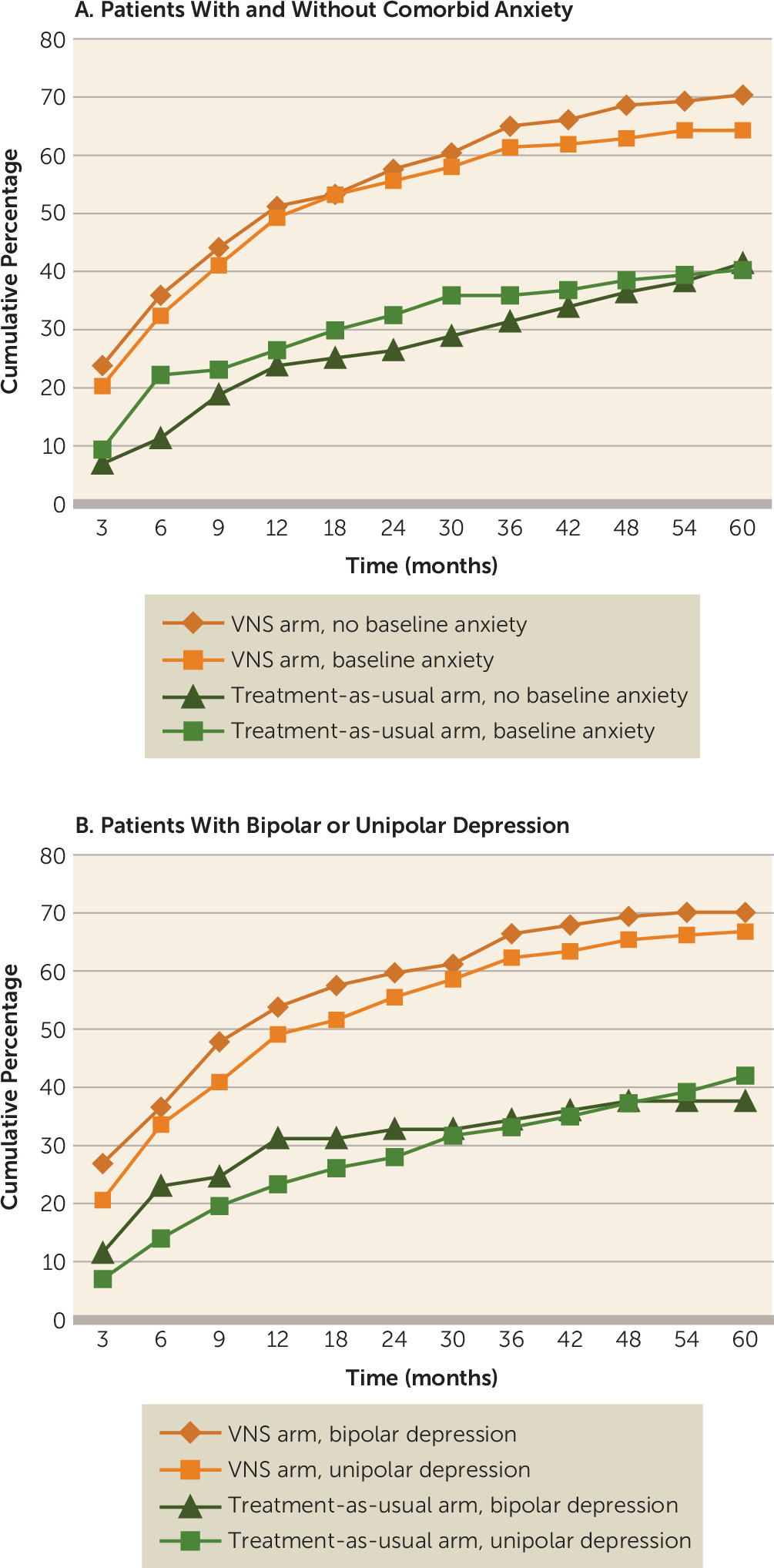
Subanalyses of cumulative percentage response rates were also performed for patients with and without a baseline presentation of comorbid generalized anxiety disorder (based on the Mini International Neuropsychiatric Interview) and patients with bipolar depression versus unipolar depression. Consistent with the findings based on the intent-to-treat population, the results of the subanalyses showed significant differences (p<0.05) within each comparator arm grouped by baseline comorbid anxiety or by unipolar versus bipolar depression; the differences were evident by 12 months and continued to 60 months (
Figure 5A and
5B).
Safety
Results based on the safety assessments of suicidality and mortality are presented below. Results related to frequency, intensity, and burden of side effects based on the patient-rated Frequency, Intensity, and Burden of Side Effects Rating scale are presented in the Supplemental Results section of the online data supplement. The safety profile based on this scale was similar between the two treatment arms, showing that adjunctive VNS does not lead to an additional side effect burden compared with treatment as usual only.
Suicide attempts and suicidal ideation.
Based on three different outcome measures of suicidality (as outlined in the Method section), both treatment arms demonstrated an improvement from baseline over the course of study participation; however, the VNS arm showed a greater reduction in the suicidality profile compared with the treatment-as-usual arm. The difference was statistically significant for QIDS-SR item 12 (odds ratio=2.11, 95% CI=1.28, 3.48; p=0.035) and the investigator-completed suicidality assessment (odds ratio=2.04, 95% CI=1.08, 3.86; p=0.029), but not for MADRS item 10 (odds ratio=1.67, 95% CI=0.98, 2.83; p=0.058).
Mortality.
All-cause mortality was markedly lower in the VNS arm than in the treatment-as-usual arm (3.53 per 1,000 person-years [95% CI=1.41, 7.27] and 8.63 per 1,000 person-years [95% CI=3.72, 17.01], respectively) (
Table 2). The rate of completed suicides was also lower in the VNS arm than in the treatment-as-usual arm (1.01 per 1,000 person-years [95% CI=0.11, 3.64] and 2.20 per 1,000 person-years [95% CI=0.24, 7.79], respectively).
Fifteen patients died during the study, including seven in the VNS arm and eight in the treatment-as-usual arm. Information on deaths and related causes is provided in the Supplemental Results section of the online data supplement.
Discussion
Findings from this long-term, naturalistic, prospective, longitudinal, multicenter, open-label, observational patient outcome registry study provide important outcome information about a patient population that is not generally studied. These were patients who continued to experience severe and chronic depression after an average of 8.2 failed treatments for depression.
As demonstrated in this study, patients in the VNS arm experienced clinically and statistically significant benefits compared with patients in the treatment-as-usual arm for most of the measured clinical efficacy outcomes. Although the indices of depressive severity at baseline suggest that patients in the VNS arm were a more severely ill group than those in the treatment-as-usual arm, the patients in the VNS arm had significantly more positive outcomes in response rate, time to response, and duration of response, while also experiencing reduced mortality and suicidality, as evident in both the clinician-rated and the patient-rated scales.
The improved outcomes with adjunctive VNS observed for both ECT responders and nonresponders is remarkable. For patients who respond to ECT―who often rely on maintenance ECT or additional courses of life-disrupting treatment with ECT―VNS may provide a tolerable alternative, and for patients who do not respond to ECT―for whom psychiatric care offers limited therapeutic options―VNS demonstrates significant efficacy.
As there is a lack of evidence-based biological treatment options for treatment-resistant depression (other than ECT), the results from this registry provide encouragement to pursue aggressive neurostimulation interventions.
There are several important limitations to our registry design. Given ethical concerns about following such a severely ill patient population over a 5-year period, the registry had a naturalistic, observational design and did not randomly assign patients to the treatment groups (
22,
23). Similarly, the treatment assignment in the registry was not blinded, in part because it would have been unethical to implant a sham device for a long duration in severely ill patients.
A robust treatment response was observed in the VNS arm, exceeding the response rate in the treatment-as-usual arm, and it is reasonable to ask whether this represents an effect in which patients have a higher expectation of therapeutic improvement with an implanted device (
24). While not exactly equivalent to an expectation effect, a potential for a “placebo” effect is diminished by the patients’ elevated baseline illness severity and chronicity (
25). It would also seem unlikely that an expectation effect would endure over several years, but this has not been studied in a trial of this duration. Also, in the ECT subanalysis, separation between groups only begins at 9 months for the responders and at 2 years for the nonresponders. It seems unlikely that an expectation effect would commence after so much time had elapsed.
Inclusion of the D-21 rollover patients in the VNS arm may be another study limitation, as the D-21 rollover patients who had a positive experience with VNS may have been more likely to participate in the registry; however, a sensitivity analysis of the VNS group (with and without the D-21 rollover patients) demonstrated similar treatment effects and similar treatment differences in comparison to the treatment-as-usual arm.
The 1-year response and remission rates in the treatment-as-usual arm were considerably higher compared with those in a study by Dunner et al. (
22) that examined the effects of treatment as usual in patients with treatment-resistant depression. Among the registry patients in the treatment-as-usual arm, the rates after 1 year of treatment were 25% for response and 12% for remission, compared with 12% and 4%, respectively, in the Dunner et al. study. It is not clear what factors contributed to the higher response and remission rates in the registry study, but it is possible that differences in baseline illness status or the frequency of visits in the registry study contributed to improved response and remission rates in the treatment-as-usual arm.
In summary, adjunctive VNS resulted in superior outcomes in both effectiveness and mortality over a 5-year period compared with treatment as usual alone for patients with a chronic, severe course of treatment-resistant depression, a patient population for whom evidence-based treatment options do not currently exist.
Acknowledgments
The authors thank the patients who participated in the study, as well as the principal investigators and study staff. The registry was sponsored by Cyberonics, Inc., through contracts to investigative sites. Statistical analyses were performed by Cyberonics and reviewed by the senior author. Karishma Manzur, Ph.D., of Lenimen Consulting, Inc., provided medical writing support and was compensated by Cyberonics.