Posttraumatic stress disorder (PTSD) is persistent (
1) and impairing (
2) but can be treated with psychotherapy (
3). One such effective treatment is prolonged exposure (
4), which utilizes therapeutic exposure as its primary technique for promoting recovery (
5). Formulated from emotional processing theory (
6), prolonged exposure helps the patient confront the trauma memory and real-life situations that provoke symptoms. This allows the patient to integrate new, adaptive information regarding safety from threat. Repeated exposure usually results in a reduced fear response and promotes corrective learning, whereby the likelihood and intensity of a future fear response to that stimulus is lessened (
6).
Previous PTSD psychotherapy imaging studies have observed increased prefrontal recruitment during recall of the trauma memory after treatment (
8–
10), although decreased recruitment has also been observed during trauma memory recall (
11), processing of negative (
12) and trauma-related pictures (
13), and conflict processing (
14). Similarly, increased recruitment of the anterior cingulate has been noted during processing of fearful versus neutral faces (
15) and during anticipation of negative versus positive pictures (
12), while activation in limbic structures such as the amygdala and anterior insula have shown posttreatment attenuations during recall of the trauma memory (
16), a classic (
14) and an affective Stroop task (
17), and anticipation of affective pictures (
12). Thus, the predominant pattern of experimental results is consistent with proposed mechanisms of psychotherapy—increased prefrontal recruitment and control over limbic structures involved in threat detection and emotion induction (
18). On the basis of these findings, we expected individuals assigned to receive prolonged exposure therapy to display a greater attenuation of amygdala and anterior insula activation during the processing and detection of emotional stimuli. We also expected to see increased prefrontal recruitment during all phases of emotion processing, from initial detection and processing of stimuli to deployment of automatic and effortful regulatory strategies.
Discussion
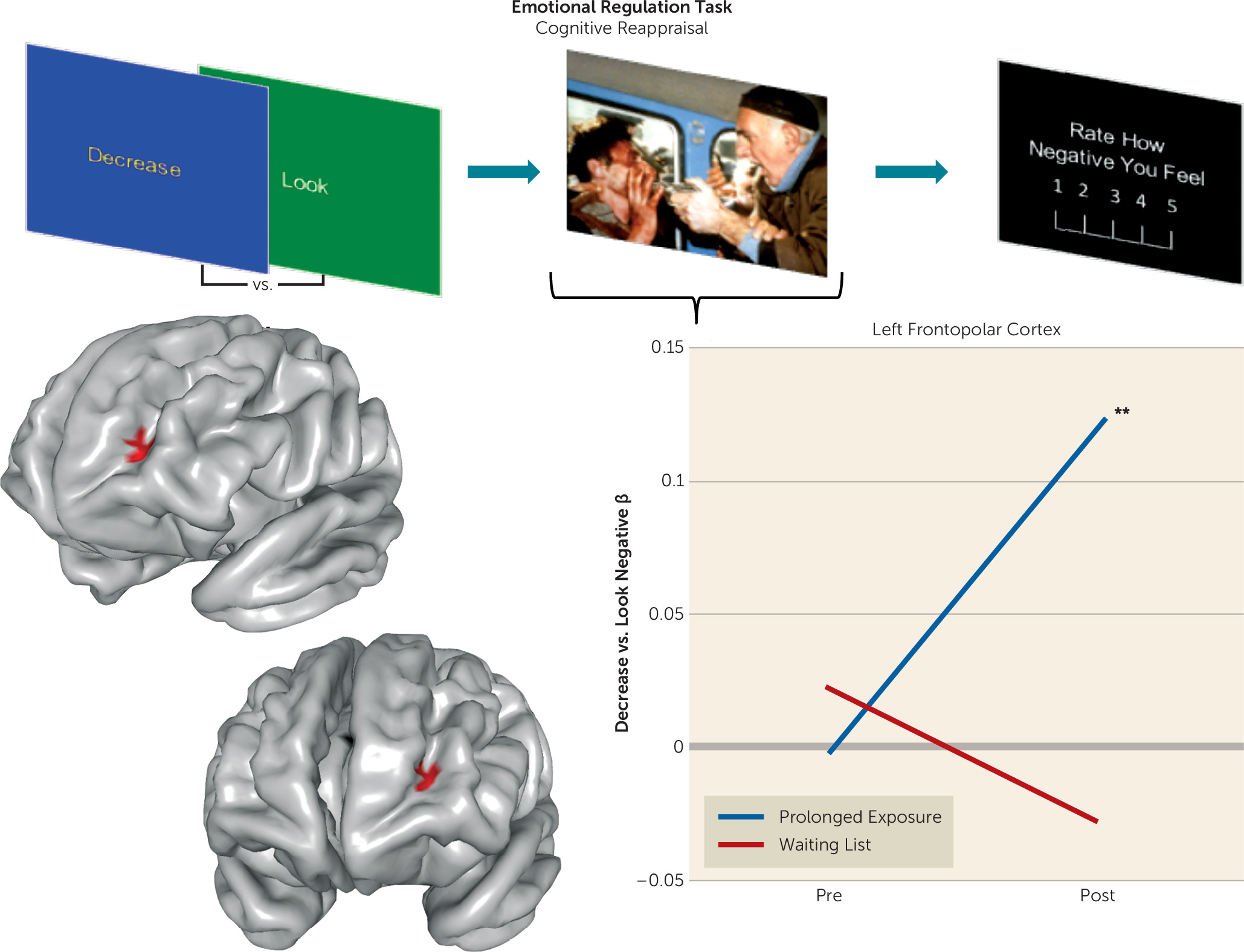
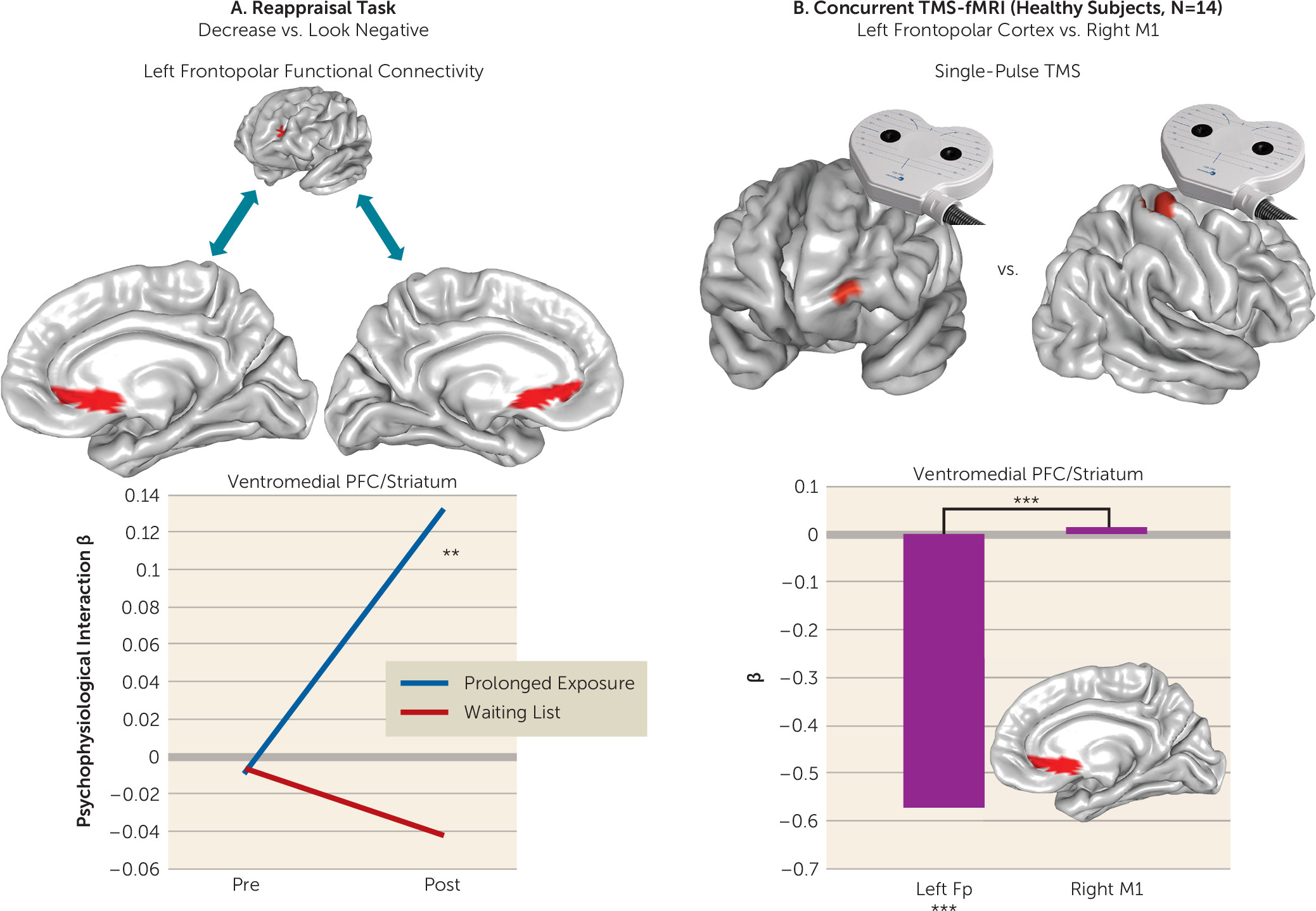
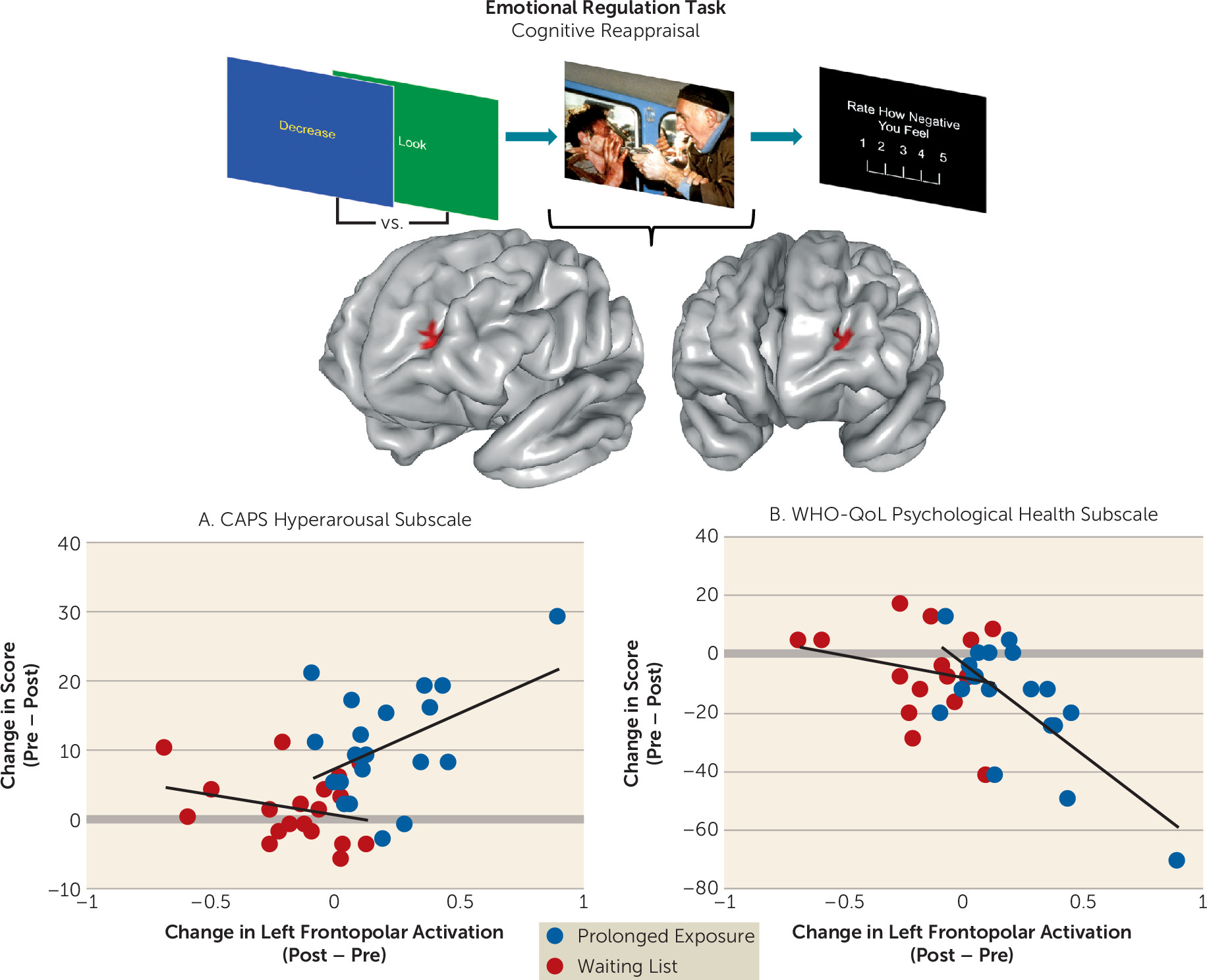
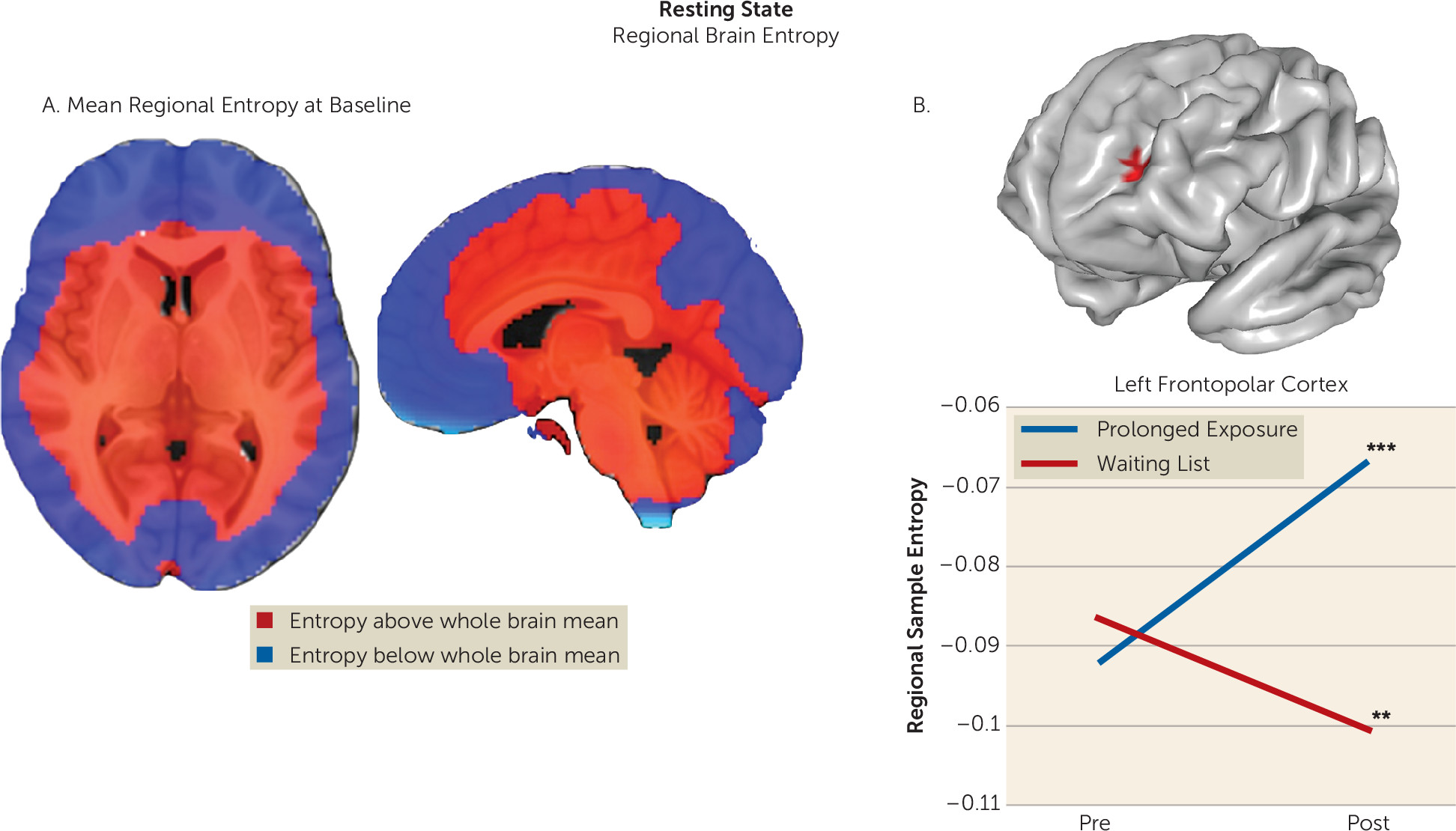
We assessed brain function in individuals with PTSD during emotional reactivity and regulation to better understand how prolonged exposure therapy conveys therapeutic benefit. No treatment-related changes were observed in reactivity to emotional cues or when regulating interference from emotional conflict. However, the left lateral frontopolar cortex displayed increased activation and increased connectivity with the ventromedial prefrontal cortex/ventral striatum during cognitive reappraisal after treatment. Concurrent TMS-fMRI in healthy participants demonstrated that frontopolar cortex stimulation modulates downstream activity in this connectivity target. Increases in frontopolar activation were related to improvement in hyperarousal symptoms and psychological well-being. Finally, the lateral frontopolar region showing activation change during cognitive reappraisal also demonstrated a wider variety of resting-state signal fluctuation patterns over time. Taken together, these findings indicate that 1) the most prominent therapeutic brain change following prolonged exposure is prefrontal rather than limbic and manifests during deliberate emotion regulation; 2) this change is clinically relevant and is related to improvement in symptoms and psychological well-being; 3) this change manifests in the lateral frontopolar cortex and its interactions with the ventromedial prefrontal cortex/ventral striatum, a recipient of frontopolar downstream influence; and 4) this change is evident during both regulation of emotion and at rest and may therefore reflect a generalized shift in frontopolar function.
These results inform a novel view of the brain mechanism of exposure therapy. In contrast to existing accounts of psychotherapy mechanisms (
18,
32), we observed no limbic attenuation during emotional reactivity, no increased recruitment of posterior lateral prefrontal substrates implicated in top-down control (
33), and no prefrontal change during emotional reactivity, emotional conflict, or emotional conflict regulation. Notably, this contrasts with treatment moderation results in this sample, wherein emotional reactivity and emotional conflict regulation–related brain function predicted treatment outcome, as reported in the companion article (
34). Instead, we demonstrate that exposure therapy alters functioning of the most anterior portion of the prefrontal cortex (BA 10) during deliberate emotion regulation, as well as its connectivity with a ventromedial corticostriatal target that is a target of its downstream influence. Together, these findings point toward a prominent, selective effect of exposure therapy on a cortical substrate that is anatomically and functionally distinct (
31) from other prefrontal cortical regions widely held to convey the efficacy of psychotherapy (
18,
35).
In contrast to prefrontal cortical regions implicated in executive control or salience (
36), the frontopolar cortex (also referred to as the anterior prefrontal cortex [
31] or the rostral prefrontal cortex [
29]) is believed to control the balance of stimulus-dependent attention (e.g., to the external environment) and stimulus-independent attention (e.g., attention toward the internal milieu) (
29). The lateral frontopolar region identified here has been implicated in higher-order processes requiring a continual integration of inner mental phenomena with outward attention to “keep something in mind” while performing concurrent tasks (
29). The frontopolar cortex is composed primarily of BA 10, a substrate with unique cytoarchitecture (
31). Substantially enlarged in humans, it is one of the last regions to mature developmentally and is almost exclusively interconnected with higher-order associative cortices involved in cross-modal information integration (
31). Hemodynamic changes in this region occur across many paradigms (
29), consistent with its proposed role as a coordinator of multiple component cognitive functions processed by more posterior prefrontal areas (
31). Meta-analytic data indicate that this region is activated by reappraisal (
37), particularly in the later temporal phases (
38), and is hypoactive in PTSD (
39), suggesting that the effects observed here may indicate normalization of an abnormality.
Increased activation in this region was concomitant with increased ventromedial prefrontal (BA 25 and 32)/ventral striatal connectivity. Activation of this ventromedial (BA 25)/ventral striatal region moderated treatment response during emotional conflict regulation at baseline (
34), illustrating a potential connection between these processes and a common substrate. BA 25, the subgenual cingulate, has been implicated in parasympathetic modulation of internal state (
40), while the nucleus accumbens/ventral striatum has been shown to mediate relationships between successful reappraisal and both ventromedial prefrontal and frontopolar function (
41). That greater activation in this region at baseline predicted more favorable psychotherapy outcomes in this sample was interpreted in the context of emotional conflict regulation as an enhanced capacity to attenuate arousal/vigilance after perturbation by a salient stimulus (
34). Consistent with the proposed role of the lateral frontopolar cortex in switching between stimulus-dependent and stimulus-independent attention (
42), this convergence suggests that psychotherapy may train the lateral frontopolar cortex to better evoke, amplify, or integrate attention toward an internal regulatory process that mediates successful emotion regulation and marks cessation of reappraisal (
41). Clinically, this may manifest as less engagement in avoidance strategies to regulate emotional state and more moderate, less excessive responses to emotionally salient stimuli.
It is noteworthy that BA 10 has demonstrated psychotherapy-related changes in two PTSD imaging studies, one showing increased left hemisphere recruitment during script-driven imagery in police officers (
10), and the other showing attenuated right hemisphere activation during anticipation of negative versus positive images in assaulted women (
12). Thus, our findings add to accumulating evidence that frontopolar cortical function conveys at least some of the beneficial effects of PTSD psychotherapy. We expand on initial findings by demonstrating change in lateral frontopolar reappraisal-related activation, connectivity with the ventromedial prefrontal cortex/ventral striatum, and frontopolar resting entropy, as well as by demonstrating that lateral frontopolar cortex stimulation can directly modulate ventromedial prefrontal/ventral striatal function. Studies in social anxiety disorder have also demonstrated functional changes in BA 10 after treatment, for example, during social evaluation after treatment with nefazodone (
43) and during threat processing after cognitive-behavioral therapy (
44). The frontopolar cortex is also an efficacious TMS site for the treatment of major depression (
45), and frontopolar cerebral blood flow indexes treatment response after exposure with response prevention for obsessive-compulsive disorder (
46). Thus, the frontopolar cortex may be a transdiagnostic therapeutic target across disorders that are characterized by diminished positive affect and exaggerated fear, anxiety, and threat reactivity.
We demonstrate the capacity for lateral frontopolar stimulation to influence ventromedial prefrontal/striatal signal in healthy individuals, which provides initial evidence for an integrated communication pathway operating in multiple contexts. This communication may therefore reflect a process of general relevance, consistent with the interactions of these regions during a range of behaviors (
28,
30) and with the proposed role of the frontopolar cortex as an attentional gate (
29). Specifically, transient lateral frontopolar activations are also thought to support bidirectional switching between stimulus-dependent and stimulus-independent processing modes (
29), which may underlie TMS-induced deactivation of the ventromedial prefrontal cortex. As this region is implicated in control and awareness of one’s internal state (
40), attenuation of regional activity here by frontopolar stimulation may signal a shift away from a stimulus-independent state of rest (
47). Likewise, increased resting entropy in the lateral frontopolar cortex after psychotherapy suggests that this region is able to function more flexibly and assume a more varied repertoire of configurations, which may reflect a wider range of mental states. Here, we utilized TMS-fMRI and resting-state data only to follow up on primary analyses of task findings, and we did not undertake an extensive investigation of these metrics. Therefore, the findings should be considered initial supporting evidence to better contextualize the results of task effects, while future investigations focusing specifically on TMS-fMRI and resting-state metrics in PTSD will provide further insights.
This study has several limitations. The first is the lack of a traumatized healthy comparison sample, which might have allowed us to determine whether functional changes reflect normalization of abnormalities or compensatory adaptations. Second, we did not collect frontopolar TMS-fMRI data in patients, which would have been most informative for this investigation. We note that the TMS-fMRI findings reported here may not necessarily apply to individuals with PTSD. Third, we did not counterbalance task order across participants, as it was not possible to ensure balanced administrations across randomized groups. However, this could also reduce generalizability of brain change effects if the task administration order exerted habituation effects on the brain dynamics in a given task that showed a differential change over time between treatment arms. It is notable that we did not detect hypothesized treatment-related changes in limbic regions (e.g., the amygdala and insula) previously demonstrated to be hyperactive in PTSD and to display changes after therapy (
14–
16). This may reflect a lack of power to detect effects of smaller magnitude. However, it is also noteworthy that among randomized controlled PTSD imaging studies, changes in prefrontal function in the absence of limbic changes have been observed with a frequency (
10,
11) equivalent to that of limbic changes (
14,
16), which may be related to variation in the experimental task, study sample characteristics, or other factors. Further studies are needed to understand these sources of variation. Additionally, that effects were observed only during the reappraisal task could be related to differences in evoked arousal related to the complex affective picture stimuli utilized in that task, as opposed to the emotional faces utilized in other tasks. However, the fact that lateral frontopolar entropy changes were observed in the immediate treatment group at rest, a low-arousal state, suggests that this is not the case. Studies examining peripheral arousal measures during task completion (e.g., skin conductance response) will be helpful in delineating whether selective effects during one task are related to evoked arousal, the mental process engaged, or both.
Despite the limitations, our findings have import for understanding the mechanism of exposure therapy by demonstrating that the most prominent functional brain change during the processing and regulation of nontraumatic emotional stimuli occurs in an anatomically distinct, higher-order frontal structure (
31). This region may also be responsible for the instantiation of a conceptually distinct process—a gating mechanism dictating the balance of awareness of the internal and external world (
29). Although additional studies are needed to further elaborate on the functional significance of the frontopolar cortex in PTSD and its change after exposure therapy, the present findings identify an underexplored anatomical brain target and pathway of influence to the ventromedial prefrontal cortex with promise for stimulation-based therapeutics and augmentation of psychotherapy effects.