Bipolar disorder is characterized by mood dysregulation associated with alterations in brain connectivity. Resting-state functional MRI (fMRI) studies in bipolar disorder have shown that disease mechanisms influence intrinsic connectivity, including that of the default mode, sensorimotor, and central executive networks (
1–
3). Imbalance between the functional connectivity of the sensorimotor and default mode networks has been directly linked with the clinical expression of bipolar disorder, as illustrated in a recent study (
2) that found the sensorimotor and default mode networks showing opposite variability patterns during depressive and manic episodes.
Bipolar disorder is highly heritable (
4), but the pathway from genetic risk to clinical symptoms is not fully understood. Siblings of patients have about a 10-fold higher risk than the general population, with the mean weighted prevalence of bipolar disorder (all subtypes included) in siblings estimated at 11.9% (
5). Disease expression and familial risk for bipolar disorder influence the functional connectivity of brain regions involved mainly in the default mode network, the mesolimbic network, and the central executive network (
1,
3,
6). These neural systems–level alterations in individuals with a familial risk of bipolar disorder are thought to occupy an intermediate location on the path from genetic risk to disorder (
3,
7). However, despite harboring risk-related features, a significant proportion of relatives remain psychiatrically well. In addition, there is emerging evidence that some measures of brain functional (
8–
10) and structural connectivity (
9,
11) appear to index the avoidance or delayed onset of bipolar disorder in unaffected siblings, suggesting that these measures may involve mechanisms that actively counteract risk. Unaffected relatives may develop psychiatric morbidity at some future point, but as long as they remain well, they may manifest protective changes in brain connectivity. We consider such measures as indicators of “resilience” in relation to avoidance or delayed disease onset.
Here we describe a graph theoretical approach that seeks to disambiguate brain features of risk, disease expression, and resilience to bipolar disorder. Graph theory models the brain as a graph with regions and their connections represented as nodes and edges; its advantage over conventional approaches is that it offers tools to evaluate brain connectivity at the global and regional levels in accordance with the multiscale nature of human brain architecture (
12). We obtained resting-state fMRI data from patients with bipolar disorder, their unaffected siblings, and demographically matched unrelated healthy individuals. This design enabled us to characterize trait-related changes in brain connectivity associated with vulnerability and disease expression in bipolar disorder. We focused on 1)
global connectivity, assessed by measures of global efficiency and characteristic path length (two measures of network integration), clustering coefficient (a measure of network segregation), and small-worldness (a measure of the balance between segregation and integration); 2)
mesoscale connectivity, assessed by network modularity over a range of spatial scales; and 3)
regional connectivity, assessed by nodal degree (a measure of a region’s putative influence on the network) and participation coefficient (a measure of the extent to which a given node connects to nodes in modules other than its own).
Discussion
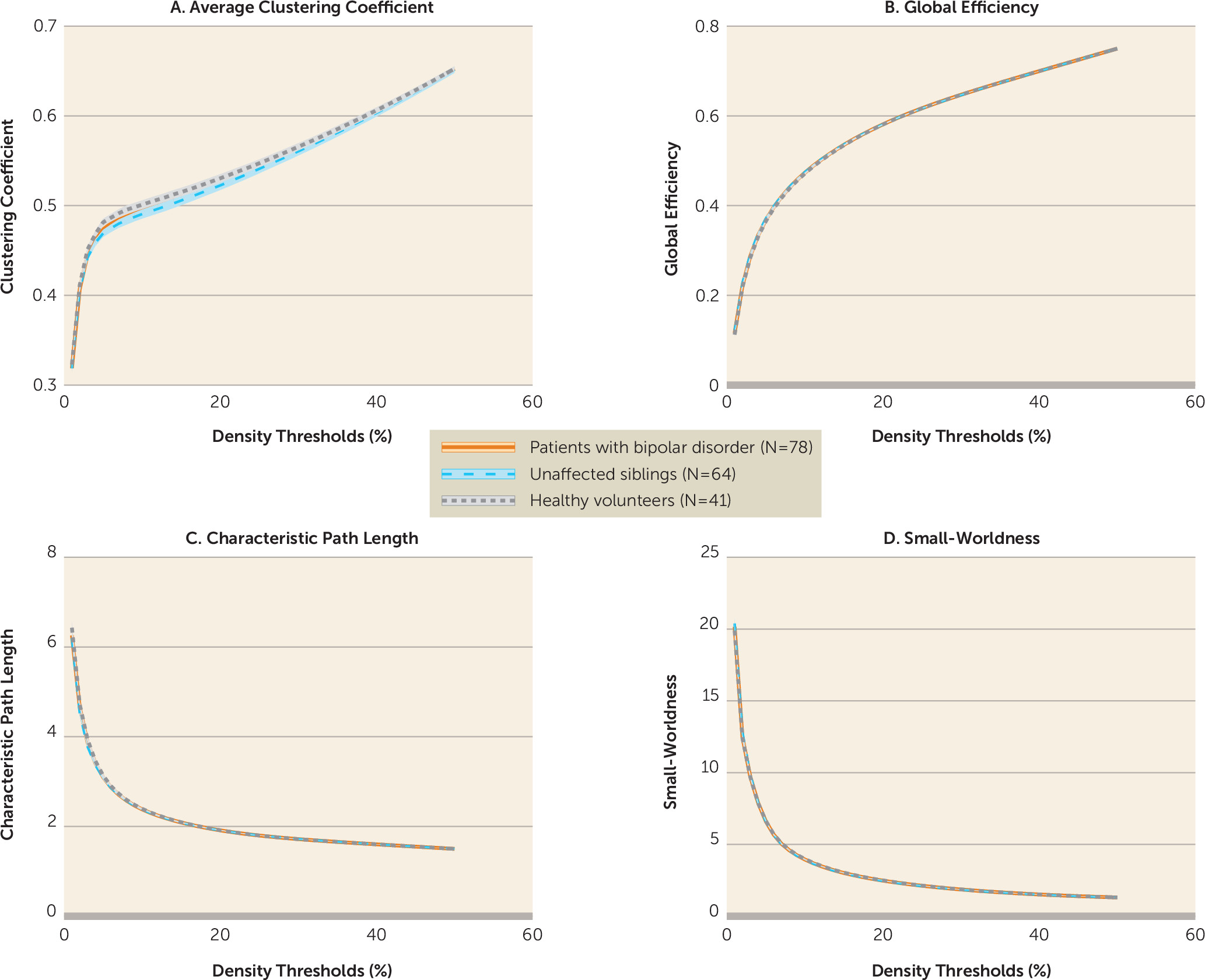
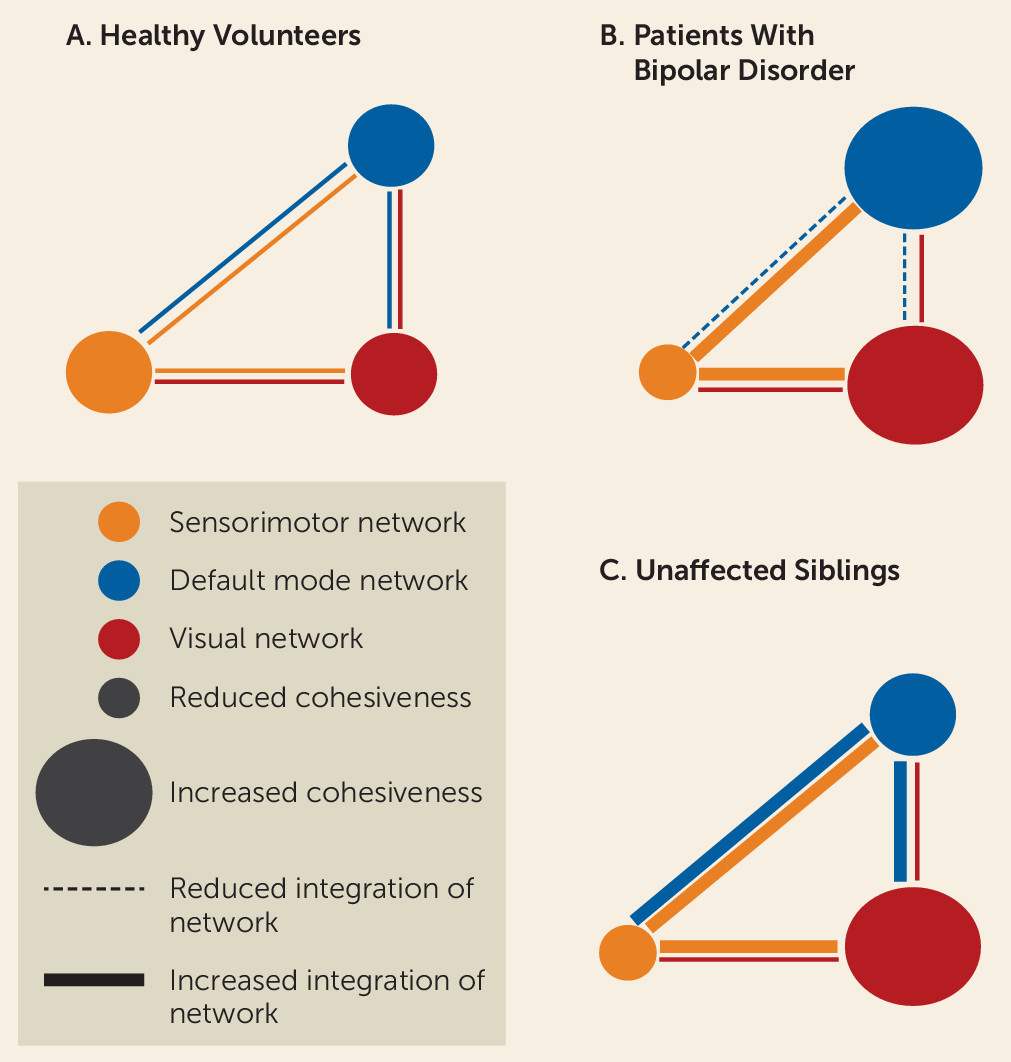
We characterized the topological organization and network modularity of the brain during the resting-state condition in patients with bipolar disorder, their unaffected siblings, and unrelated healthy volunteers. We found that disease expression, risk, and resilience to bipolar disorder were associated with mesoscale and regional changes primarily in the default mode and sensorimotor networks, while global network properties appeared to be conserved both in patients and their siblings (
Figure 4).
We found that global network properties in bipolar disorder were preserved in a manner consistent with previous studies examining the structural connectome topology (
23) and the global resting-state fMRI signal power and variance in patients with bipolar disorder (
24). This contrasts with findings in schizophrenia, where increased global signal variance and network randomization have been reported in patients (
24) and their unaffected relatives (
25). Although schizophrenia and bipolar disorder have overlapping clinical phenotypes and genetic risk factors (
4,
26), preserved global brain organization in bipolar disorder may represent a major difference between the two disorders. This observation aligns with replicated reports of preserved premorbid and academic performance in patients with bipolar disorder (
27) and superior academic performance in their relatives (
28), which is not the case for schizophrenia (
27,
28).
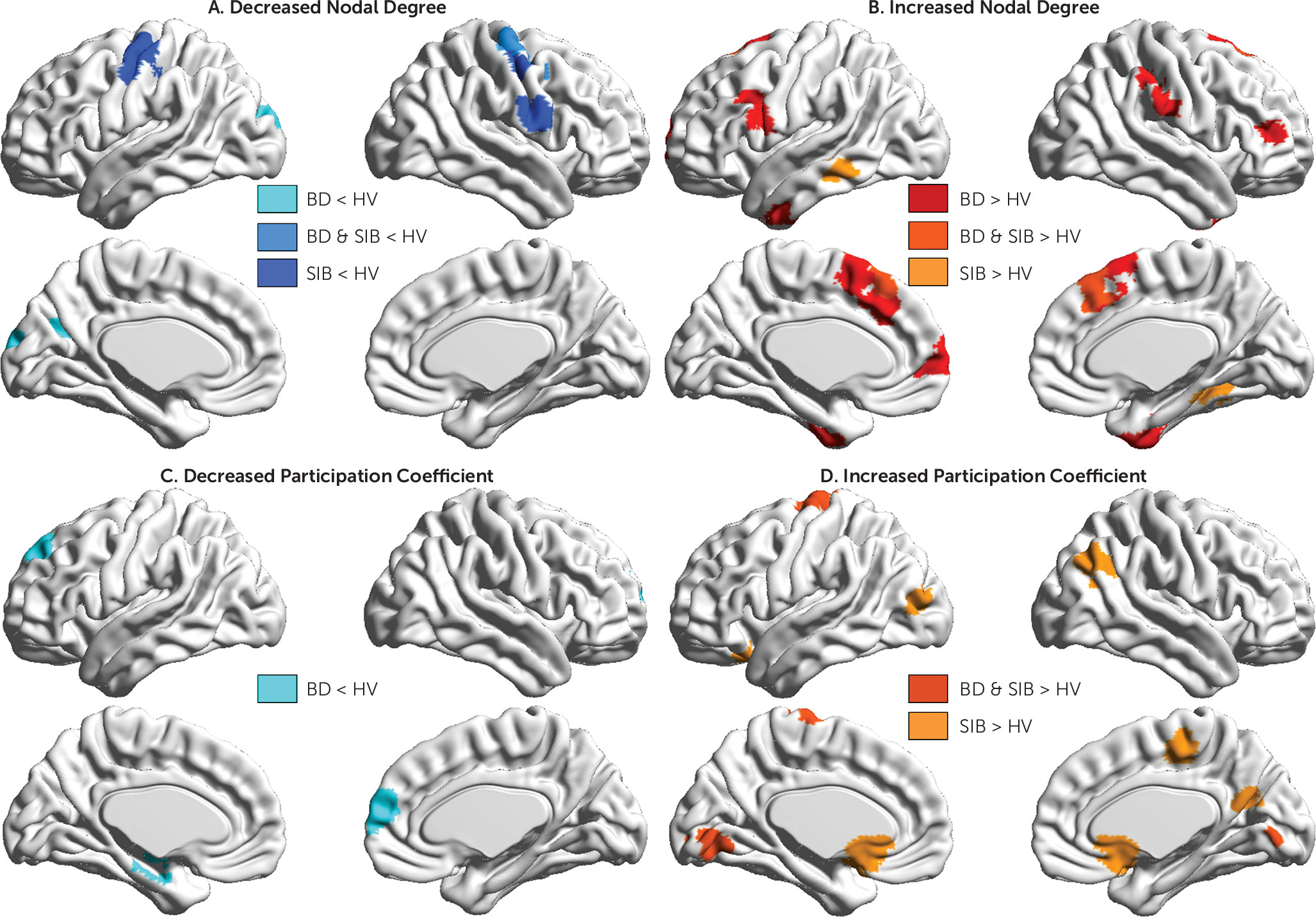
Our results suggest that mechanisms related to vulnerability to bipolar disorder affect brain organization at the mesoscale and at the regional level and that they particularly disrupt the connectivity of sensorimotor regions. Typically the sensorimotor network shows high within-system connectivity and relatively low integration with the rest of the brain (
20). This connectomic signature is characteristic of “cohesive provincial” networks that are dedicated to the specialized processing of sensory stimuli and motor responses. In the present study, patients and relatives showed decreased within-module connectivity (indicative of reduced network cohesiveness) coupled with increased interaction of sensorimotor regions with regions outside their module (indicative of greater integration). In other words, the sensorimotor network in bipolar disorder behaves as an incohesive connector network which likely disrupts the processing of sensorimotor information within the brain. It is surprising that research to date has largely overlooked the evidence for sensorimotor dysfunction in bipolar disorder given that abnormalities in motor and sensory processing are included in the diagnostic criteria for manic and depressive episodes. Complementing our findings, a large recent study (
29) has reported reduced intracortical myelination in patients with bipolar disorder predominantly in the sensorimotor regions. Further studies have found abnormally reduced motor coordination, sensory integration, sensory gating, and selective attention in patients with bipolar disorder and their unaffected relatives (
30,
31). In our data, increased motor impulsiveness was also associated with disease expression and risk for bipolar disorder, consistent with the connectivity changes in the sensorimotor network observed in patients and siblings.
The findings of this study underscore the role of regional default mode network connectivity in disease expression and resilience to bipolar disorder. Regional connectivity was evaluated using nodal degree and participation coefficient. Nodal degree is a measure of general connectedness, and participation coefficient is a measure of intermodular integration of individual brain regions. Typically, the default mode network regions show high within-network cohesiveness (high nodal degree) coupled with high between-network integration (high participation coefficient) (
20,
32). These properties allow the default mode network to act as a “cohesive connector” and thus support a wide range of brain functions (
20,
32). Consistent with previous reports of default mode network dysconnectivity in bipolar disorder (
33,
34), we found that patients had abnormally reduced intermodular integration of two core regions of the default mode network—the ventromedial prefrontal cortex and hippocampus. In healthy individuals, hippocampal-midbrain connectivity supports reward-based learning and memory, and hippocampal-amygdala connectivity underpins contextually appropriate assignment of affective value and behavioral response selection (
35). A similar role is conventionally attributed to the ventromedial prefrontal cortex in the regulation of the evaluative, expressive, and experiential aspects of emotion (
36). Reduction in the integrative role of the hippocampus and ventromedial prefrontal cortex likely leads to impairment in stimulus appraisal and in contextually appropriate response selection. Moreover, the reduction of the integrative role of the default mode network, when considered together with the findings of the sensorimotor network dysconnectivity described above, supports the study by Martino et al. (
2), who found that the balance between activity patterns of the default mode and sensorimotor networks was altered during acute episodes, favoring the default mode network in depression and the sensorimotor network in mania. Our observations suggest that a dysfunction of the default mode and sensorimotor networks is also present during interepisode intervals. The characterization of the cellular correlates of reduced default mode network integration is beyond the resolution of fMRI. Nevertheless, the present results are congruent with impaired neuronal plasticity and may be causally linked to the reduction in GABAergic and glutamatergic receptors reported in postmortem studies of bipolar patients (
37,
38).
In contrast to patients, siblings had high participation coefficients in multiple regions of the default mode network that were even higher than those of the healthy volunteers. We infer that enhanced integration of the default mode network may represent an adaptive response to the dysconnectivity of the sensorimotor network observed in siblings and may thus be conducive to resilience (or at least delayed illness onset). This interpretation is speculative pending confirmation by longitudinal studies. Although one might consider increased integration of the default mode network as a prodromal marker for bipolar disorder, this is unlikely given the low risk for conversion in siblings, as discussed earlier, and in greater depth in the data supplement.
We took great care in minimizing potential head motion artifacts based on strict inclusion criteria, in addition to regressing out the average head motion from all of our metrics. The construction of brain graphs from fMRI data requires multiple methodological choices. We confirmed the robustness of our key results using alternative approaches, as detailed in the data supplement (including in Figure S6). Longitudinal studies of high-risk individuals are needed to confirm the association between default mode network connectivity and resilience and to test whether the clinical symptoms of bipolar disorder arise as a result of failure to either develop or maintain enhanced default mode network integration.
In summary, we found that the pathophysiology of bipolar disorder is characterized by abnormalities in regional—and not global—network properties, mainly affecting the default mode and sensorimotor networks. Enhanced default mode network integration was observed in unaffected siblings, which may have mitigated the effects of risk-related dysconnectivity in the sensorimotor network. The latter observation has the potential to open up avenues toward uncovering protective neural mechanisms and thus inform novel interventions to prevent or modify the course of bipolar disorder. Studies in neurological patients (e.g.,
39) and healthy individuals (e.g.,
40) suggest that both the default mode and sensorimotor networks may be amenable to plasticity-enhancing interventions. Future empirical studies may help define the types of plasticity-dependent interventions that might prove useful in bipolar disorder based on the spontaneous adaptive changes seen in resilient relatives.