There is a fierce urgency to revolutionize our approach to treating depression and get the right treatment to the right person at the right time. One person’s depressive illness may arise for different biological reasons than another’s. There are hundreds of combinations of symptoms that can produce a diagnosis of depression, but they do not direct nor predict response to any particular treatment. As a result, getting the right treatment quickly enough to recover typically occurs by chance. It often takes many years of trying one treatment option after another, based on the clinician’s experience and on “waiting and seeing” after each trial (
1). This process leads to chronic disability and often to treatment resistance (
2). It isn’t surprising that someone with depressive disorder might consider suicide after months and months of failed treatment, with no relief in sight.
Right now, almost 60% of patients fail to recover after a single antidepressant trial, and 20% fail to respond to any intervention (
3,
4). The explosion of insights from neuroscience have renewed hope for developing assays that will guide the choice of intervention that is most effective for each patient from the beginning of their treatment. Such personalized treatment may pay off more quickly if we anchor our search on the organ of interest, the brain. In vivo functional brain imaging and functional connectivity assays offer a direct window into the brain in action and a means to quantify patient-level dysfunctions that are relevant to personalizing treatment (
5).
In this issue of the
Journal, Dunlop et al. (
6) report that the extent of functional connectivity of the subcallosal cingulate cortex distinguishes which patients are likely to remit following cognitive-behavioral therapy (CBT) and which patients are unlikely to respond to antidepressants. Focusing on the cingulate was a logical choice. The authors have established the relevance of this region to depression treatment (
5), and the subgenual cingulate is a hub region in the brain circuits that are disrupted in this disorder (
7). Resting-state functional MRI scans were undertaken for 122 patients with major depressive disorder who had at least moderate symptoms and who were part of the Emory Predictors of Remission in Depression to Individual and Combined Treatments Study (
7). A strength of the study is that patients were treatment naive at the time of pretreatment baseline scanning. Patients were then randomly assigned in an equipoise manner to receive 12 weeks of treatment with CBT or with one of two types of antidepressants: the selective serotonin reuptake inhibitor (SSRI) escitalopram or the serotonin norepinephrine reuptake inhibitor (SNRI) duloxetine. Treatment outcomes were evaluated by comparing remitters, defined by a 17-item Hamilton Depression Rating Scale (HAM-D) score ≤7 at weeks 10 and 12, to nonresponders, defined by a <30% improvement in symptoms on the 17-item HAM-D. This approach excluded patients with midrange symptom improvements, considered partial responders. Of the initial 122, 58 patients remitted, and 24 were nonresponders. Prior to treatment CBT remitters were characterized by heightened connectivity of the subcallosal cingulate with three other regions: the ventrolateral/insula prefrontal cortex, dorsal midbrain, and left ventromedial prefrontal cortex. By contrast, antidepressant nonresponders were characterized by comparatively less connectivity of the cingulate with these regions. Because there were comparatively few nonresponders to antidepressants, this finding of less connectivity was revealed with SSRI and SNRI arms collapsed and was similar within each drug arm. Predictive receiver operating characteristic models showed that the summed connectivity across these regions classified CBT remitters with around 78% accuracy and antidepressant nonresponders with around 75% accuracy.
Personalizing treatment will unfold in steps. How far does this study take us, and what are the next steps? The receiver operating characteristic models yielded a promising accuracy, especially when compared with the current trial and error approach. The input connectivity features survived permutation testing of subsamples. We now need to establish the robustness of these models in studies powered for cross-validation, through replication in new samples and in prospective tests of the cingulate connectivity metric.
Future clinical application requires that findings such as these can be applied to an individual patient. Such future application also relies on actionable connectivity metrics that predict discrete intervention options. A promising aspect of the Dunlop et al. findings is that clear positive connectivity above a set cut-off would translate to a recommendation for CBT, while clear negative connectivity below a set cut-off would translate to a recommendation for antidepressants. Since there is overlap in the cut-offs, we need to understand how to interpret midrange connectivity.
The translational relevance of using imaging as a biomarker for guiding treatment will also depend on the incremental value of imaging biomarkers in the armamentarium of therapeutic options. Another important aspect of the Dunlop et al. study is the inclusion of a nondrug treatment that may be attractive to many patients who either do not want to take antidepressants or who do not respond to them. Physicians, including at the front lines, want actionable objective tests that can help guide their choice of when to prescribe versus when to refer for CBT.
These early successes are based on random assignment to treatment and then identifying which pretreatment imaging dysfunctions predicted responders from nonresponders. This is also true of other large biomarker trials such as the Establishing Moderators and Biosignatures of Antidepressant Response for Clinical Care study (
8) and the International Study to Predict Optimized Treatment in Depression (
9). The promise of the findings for getting depression treatments right the first time highlights the need for prospective trials that use “brain type” and not random chance trialing of treatments, as well as use a wider array of treatments suited to multiple brain dysfunction profiles.
Findings such as those exemplified by Dunlop et al. (
6) are encouraging because they indicate that we can identify brain imaging biomarkers for predicting with precision which patient is likely to benefit from which treatment and why. We have the opportunity to drill down into the “why” and understand the mechanisms by which a particular metric, such as the subcallosal cingulate connectivity, confers an advantage for a particular treatment in one patient but not in another. In other words, we have the opportunity to develop clinically applicable biomarkers that also have an understandable basis in the brain basis of depression. As an analogy, cardiovascular medicine has faced similar challenges in translating complex biological features into personally applicable clinical markers (
10), but with concerted effort over the past few decades have moved ahead with a typology that links different dysfunctions in the organ of interest to specific treatment indications. Heart MRI, in the toolkit of multiple imaging modalities, helps differentially diagnose birth defects, tumors, and other heart failure problems, and guides choice of treatments, including lifestyle changes, medications, surgery, and their combination. In considering this analogy for brain MRI and depression, an important future direction will be to consider how specific dysfunctions, such as in cingulate connectivity, can be distinguished from or interact with other brain circuit dysfunctions, and thus flesh out the details of how a brain typology maps onto clinically viable indicators such as suitability to different treatment interventions (
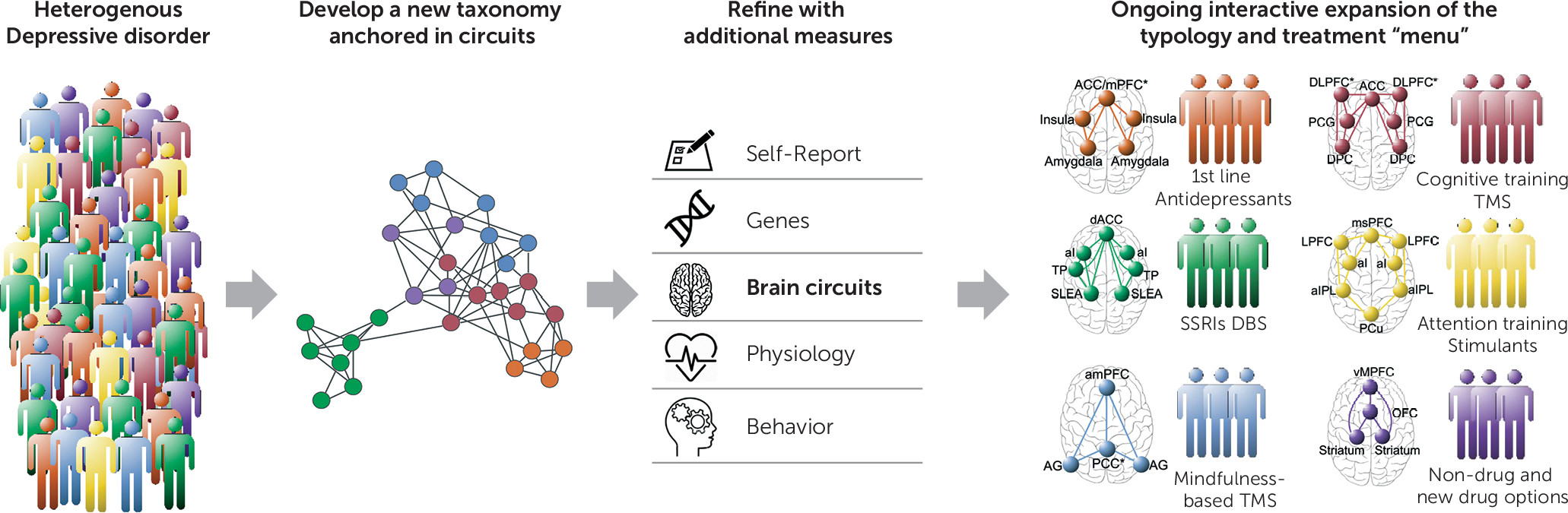
Figure 1).
With a precision approach that connects neuroimaging insights directly with clinically actionable treatment outcomes, we have the opportunity to improve and save the lives of many. We now have early successes. Our national and global imperative is to now leverage these early successes to address the yawning gap between research insights and their translation into prospective trials and real-world practice.