The ability to experience pleasure during the anticipation and receipt of reward is a fundamental human experience and is central to the drive to survive and thrive. Deficits in this domain have been identified in both mood and substance use disorders (
1,
2). The symptom of anhedonia—the inability to enjoy previously pleasurable or rewarding activities—is a core feature of depressive disorders. Abnormalities in reward processing have been identified from observational, behavioral, and neurobiological studies as a potent risk factor for the emergence of depression as well as substance use disorders (
3).
Both human neurobiological studies and experimental animal studies have localized key brain regions involved in experiences of reward (
4). This combined body of data validates the central role of the orbitofrontal cortex and the striatum in neural responses to reward. The orbitofrontal cortex plays a role in processing sensory input and in estimating the value of experiences of reward, including natural (e.g., gustatory), monetary, and drug-related incentives (
5,
6). The striatum drives appetitive behavior by facilitating incentive salience (e.g., wanting) and reward-related learning (
7). While orbitofrontal cortical and striatal volumes show normative increases and then decline beginning in early adolescence (
8), reductions in the volumes of these structures have been identified in adolescent depressive disorders in relation to the symptom of anhedonia (
9–
11). Variations in orbitofrontal cortical and striatal volumes have also been linked to substance use and later substance use disorders. Numerous studies have documented decreased gray matter volume in the orbitofrontal cortex in substance-using individuals, including those using alcohol (
12,
13) and marijuana (
14–
17). While the role of the striatum in “wanting” is evident in reward-related behaviors such as substance use and substance use disorders, the role of the orbitofrontal cortex may be related to the pursuit of goal-directed behaviors, or drive, which may evolve into habitual actions (
18,
19), as well as learning associations between stimulus and reward (
2), both highly relevant to risk for substance use disorders.
Of particular interest, associations between orbitofrontal cortical and striatal structure and substance use have been found during the reward-salient period of adolescence. In the United States, alcohol and marijuana are among the first substances to be used by Caucasian and African American youths, respectively, with onsets peaking between ages 15 and 17 (
20,
21). In addition, epidemiological studies suggest that childhood internalizing problems may modify risk, positively and negatively, for onset of substance use (
22). The literature thus supports the hypothesis that anhedonia-related changes in brain maturation may have an impact on the onset of alcohol and marijuana use. Both the orbitofrontal cortex and the ventral striatum have been investigated in the context of adolescent substance use. For marijuana, while most studies have conceptualized orbitofrontal cortical volumetric alterations as a consequence of heavy use (
23), one longitudinal study (
15) found that smaller orbitofrontal cortical volume at age 12 was related to onset of marijuana use by age 16. A few studies have linked orbitofrontal cortical structure and adolescent alcohol use (e.g.,
24). Similar to the marijuana findings, one adolescent co-twin-control study (
25) reported an association of lower lateral orbitofrontal cortical volume with alcohol use. However, as no volumetric differences were found within pairs of twins with varying levels of alcohol use, these findings suggest that changes in orbitofrontal cortical volume may be a preexisting marker of risk and not a consequence of prolonged alcohol exposure. A link between structural differences in the striatum and marijuana and alcohol use is less clearly established, with some studies suggesting that ventral striatal volume increases in users (e.g.,
26). These findings suggest that the orbitofrontal cortex and ventral striatum are key areas involved in adolescent substance use and that while they may be vulnerable to the psychoactive effects of drugs, variations in their maturation may also precede and contribute to the onset of substance use.
While the key roles of the orbitofrontal cortex and the striatum in reward processing are relatively clear, how structural maturation of these regions during childhood relates to variation in hedonic tone, experiences of anhedonia and later reward-seeking and substance use, and depression outcomes remains unknown. Early deficits in hedonic tone may co-occur with or influence the developmental trajectory of the orbitofrontal cortex and striatum. In turn, these neurobiological alterations may influence later reward-related drive behavior and related disorders during adolescence. One hypothesis is that anhedonia through middle childhood leads to increased goal-directed, reward-seeking behavior, potentially compensatory, to obtain high hedonic valence experiences in the form of substance use in adolescence. Alternatively, the growth trajectory of the orbitofrontal cortex has been shown to relate to risk for later substance use, independently of anhedonia (
15), suggesting that independent processes between orbitofrontal cortical volume and adolescent substance use are also operative. Based on these established brain-behavior relationships, an investigation of the developmental trajectory of the striatum and orbitofrontal cortex, as a function of anhedonia in relation to risk for later depression or substance abuse, is of interest. Building on the literature, this study aimed to test hypotheses about how behavior and brain in these domains change together or independently influence each other to predict the risk trajectory for later substance use or depression.
Utilizing data from a longitudinal neuroimaging study of children with early-onset depression and healthy comparison subjects who were followed from preschool into adolescence, we examined the developmental trajectory of orbitofrontal cortical and striatal volumes and how they varied as a function of anhedonia ratings. While this study sample was not specifically designed to investigate risk for substance use and substance use disorder, it provided an ideal opportunity to investigate the trajectory of brain change in the context of varying anhedonia and depression across childhood to inform risk for first onset of substance use disorder in adolescence and risk for a later recurrence of depression. We hypothesized that orbitofrontal cortical and striatal volumes would decrease over time as a function of increased anhedonia even when other depressive symptoms were controlled for. Based on an extensive literature demonstrating the role of the orbitofrontal cortex and ventral striatum in onset of alcohol and marijuana use, the first substances to be used by most U.S. youths, we then sought to explore whether these brain-related volumetric trajectories predicted later substance use and depression recurrence as the study sample entered adolescence, which is a key period of risk for onset of substance use and depression. To examine the role of anhedonia in the associations with substance use, we compared whether volumetric alterations related to, and independent of, anhedonia were equally predictive of alcohol and marijuana use during adolescence.
Method
A total of 305 children (54% white, 33% black, 13% other) ages 3–6 at baseline, oversampled for symptoms of depression, were recruited in the St. Louis metropolitan area for participation in a longitudinal study of preschool-onset depression. After a first, behavioral-only study phase, healthy children and those with a history of depression (N=211) were invited to participate in further school-age and adolescent follow-up to undergo neuroimaging and behavioral and diagnostic assessments comprising three additional waves. A total of 193 children had usable relevant data from one or more scan waves (113 had data from all three scans). Proximal to the scan, children participated in behavioral assessments that included parent report (for children under age 8) on the Preschool Age Psychiatric Assessment (
27) and the Child and Adolescent Psychiatric Assessment (
28) (parent report at age 8 and parent and child report at ages 9 and over), as well as the Children’s Depression Inventory (CDI) (
29), which was administered at the time of scan. Demographic, psychosocial, and developmental characteristics were also assessed. Approximately 3 years after scan 3, participants 13–18 years of age returned for an assessment that included a diagnostic interview as well as the Composite International Diagnostic Interview (CIDI) (
30) to assess substance use. All study methods were reviewed and approved by the Washington University School of Medicine Institutional Review Board. Written assent was obtained for children at age 6 and written consent at age 16. Written consent was obtained from legal guardians at all study waves.
Assessments
Anhedonia.
Anhedonia was assessed by parent report on the Preschool Age Psychiatric Assessment at baseline and by child report on the eight-item anhedonia subscale of the CDI at each scan. Cronbach’s alpha values for the subscale were 0.608, 0.648, and 0.540 at scans 1, 2, and 3, respectively. The CDI has been shown to make valid distinctions between clinical and nonclinical levels of anhedonia (
31).
Alcohol and marijuana use.
Use of alcohol and drugs was assessed approximately 3 years after scan 3 using the CIDI. Frequency of alcohol use in the past 12 months (never, less than once a month, 1–3 days/month, 1–2 days/week, 3–4 days/week, nearly every day, every day) was assessed using three items: frequency of ≥1 drink, frequency of ≥5 drinks, and frequency of intoxication. Frequency of marijuana and other drug use in the past 12 months was assessed using similar response categories but only related to use ≥1 time. To cover the general liability to early substance use during adolescence and to capture the first instance of substance use in our major demographic groups (i.e., blacks and whites), a variable representing frequency of alcohol use and/or marijuana use was created by summing the categorical marijuana use frequency variable (0=never, 1=3 or fewer days/month, 2=more than 3 days/month) with the categorical frequency of ≥5 drinks of alcohol (alcohol use) variable (0=never, 1=less than 1 day/month, 2=1 or more days/month). We chose a higher level of alcohol consumption to capture nonnormative drinking in this population and to exclude the possibility that respondents might refer to occasional drinking during family celebrations or holidays or subjective evaluations of sensations of intoxication. However, secondary analyses examined combined alcohol/marijuana use variables at other degrees of alcohol use (i.e., frequency of ≥1 drink and of intoxication).
Other time-varying covariates.
Depression core score without anhedonia, prior to each scan (i.e., time-varying), was calculated as the number of the eight core depression symptoms (without anhedonia) endorsed by the parent and/or child.
Neuroimaging
Participants underwent neuroimaging in a 3.0-T Tim Trio (Siemens) scanner. The same scanner was used for all three scans. The two results of magnetization-prepared rapid acquisition gradient echo scans were assessed visually, and the best one was selected for further processing by blind raters. The selected magnetization-prepared rapid acquisition gradient echo image for each wave was processed using the longitudinal stream in FreeSurfer, version 5.3 (surfer.nmr.mgh.harvard.edu) (
32). Several processing steps, such as skull stripping, Talairach transformations, and atlas registration, as well as spherical surface maps and parcellations, were initialized with common information from an unbiased within-patient template. This longitudinal stream reduces the bias that would otherwise be present in selecting a single scan result as baseline, and it significantly increases reliability and statistical power. For striatal volume, we focused on the sum of left and right caudate, putamen, and nucleus accumbens volumes. For orbitofrontal cortical volume, we aggregated left and right medial and lateral orbitofrontal parcels from the Desikan atlas, one of the atlases available and validated in the FreeSurfer structural processing program (
33). To account for whole brain volume, orbitofrontal cortical and striatal volumes were regressed on whole brain volume minus orbitofrontal cortical and striatal volumes, and the residuals were then utilized as the dependent variables in analyses.
Analysis
Anhedonia and trajectories of orbitofrontal cortical and striatal volumes.
We fitted the data to a longitudinal multilevel linear model in SAS, version 9.3, to determine whether child-reported anhedonia ratings at the three scan waves (centered at the mean value of 43.5) were significantly associated with the trajectory of residualized orbitofrontal cortical or striatal volumes across time points. We chose to use multilevel linear models for three reasons: the ability to model nonindependence across observations, the ability to include categorical and continuous predictors at any level, and robustness to missing data. Given our longitudinal sample, multilevel linear models allowed us to model both between- and within-person variation simultaneously, whereas generalized linear models account for fixed, but not random, effects (
34). The multilevel linear models included random intercept and slope components with an unstructured covariance structure. Influence statistics for the multilevel linear models were obtained, and one subject with an extreme restricted likelihood distance value for the multilevel linear model of residualized orbitofrontal cortical volume was excluded from the orbitofrontal cortex analyses. Time was defined as age at scan (centered at the median age of 12), and an age-squared variable was included in the models to account for quadratic slopes. The influence of anhedonia was modeled as an interaction with age. Other covariates were sex and depression core score (without anhedonia) at the scan wave (centered at the mean of 1.85). An interaction between depression core score and age was included in the models to account for differences in orbitofrontal cortical or striatal values over time due to depression severity. An online calculator (
35) was used to identify the age bands in which the age-by-anhedonia interaction significantly predicted volumetric change. As a follow-up to the significant volume results for the orbitofrontal cortex, we used the same models to examine thickness and surface area to determine which component contributed to the volume effects. A Bonferroni-corrected p value of 0.025 (for orbitofrontal cortex and striatum) was used to assess significance.
Trajectories of brain volume and substance use at follow-up.
In brain regions for which a significant anhedonia-by-age interaction was identified, individual subject intercepts and slopes (i.e., residualized for covariates and age-by-anhedonia effects) were extracted and investigated as predictors of alcohol and marijuana use frequency 3 years after scan 3, as well as a variable representing use of either substance to represent the first substance used by youths in our sample. Because more than 60% of the 135 subjects reported no alcohol or marijuana use in the past 12 months, zero-inflated Poisson regression models were utilized to simultaneously estimate a logistic regression of the zero-inflated component of the outcome (no alcohol or marijuana use, or abstinence) and a regression of the continuous component of the outcome (frequency of alcohol or marijuana use) on the predictors. Zero-inflated Poisson regression is ideal for constructs such as substance use that contain excess zero-count data (e.g., 0=no alcohol/marijuana use), as using standard ordinary least squares regression on this type of data is likely to bias results by confounding onset of use (zero versus nonzero values) and progression (range of use within users). Zero-inflated Poisson regressions account for any overdispersion due to an excessive amount of zeros without biasing the parameters or standard errors (
36–
38). There are two components to the model; the first employs a binary distribution to generate structural zeroes, and the second generates counts, some of which may be zero. Race, sex, and age at last assessment were included as covariates. To examine whether anhedonia played a critical role in the relationship between change in brain volume and substance use, analyses were redone using intercepts and slopes extracted from an orbitofrontal cortical model that did not include anhedonia as a main effect or interaction (age-by-anhedonia) term.
In supplemental analyses, we examined whether age at first substance use was associated with orbitofrontal cortical volume or thickness. Age at onset was coded as self-reported age at first use of alcohol or marijuana or age at the first assessment when a nonzero value was reported for use. To account for the possibility that some never-users may be censored (i.e., have not had the opportunity to use the substance because of their current age), age at final assessment was used in place of age at onset for never-users. Additional supplemental analyses also examined alternative definitions of alcohol use (i.e., marijuana use with alcohol use ≥1 drink frequency and marijuana with alcohol intoxication frequency).
Trajectories of brain volume and depression at follow-up.
Depression severity at the later follow-up was also investigated as an outcome. Individual subject intercepts and slopes were included as independent variables in linear regression models of depression severity (number of depression core symptoms), covarying for race, sex, and age.
Results
The characteristics of the sample are summarized in
Table 1.
Anhedonia and Trajectories of Orbitofrontal Cortical and Striatal Volumes
The anhedonia-by-age interaction was significant in the multilevel linear model of orbitofrontal cortical volume (residualized for whole brain minus orbitofrontal cortex) (
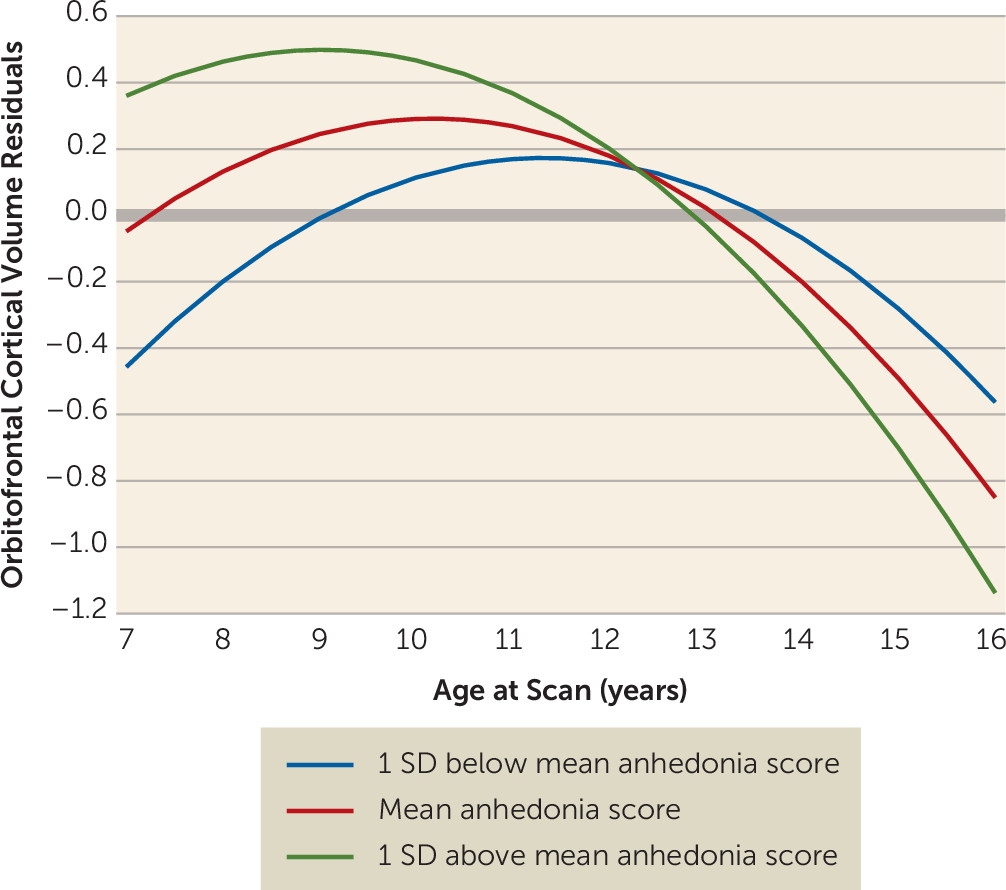
Table 2). Linear and quadratic slopes were significant. The interaction between time-varying anhedonia and age at scan was significantly associated with orbitofrontal cortical volume, with higher levels of anhedonia indicating steeper decline in volume with age. The age-by-anhedonia term was significantly related to orbitofrontal cortical volume prior to age 11.25 and after age 14.88.
Figure 1 illustrates estimated trajectories of orbitofrontal cortical volume at three values of anhedonia (the mean and one standard deviation above and below the mean). Participants with higher anhedonia scores had higher orbitofrontal cortical volumes prior to age 11.25 than those with lower anhedonia scores, even though volumetric decreases occurred across all levels of anhedonia. There was a crossover effect, with those with higher anhedonia scores showing steeper decreases. Orbitofrontal cortical thickness, but not surface area, showed significant effects similar to those for volume (
Table 2), including an association with the age-by-anhedonia interaction, with higher levels of anhedonia associated with a steeper decline in orbitofrontal cortical thickness (
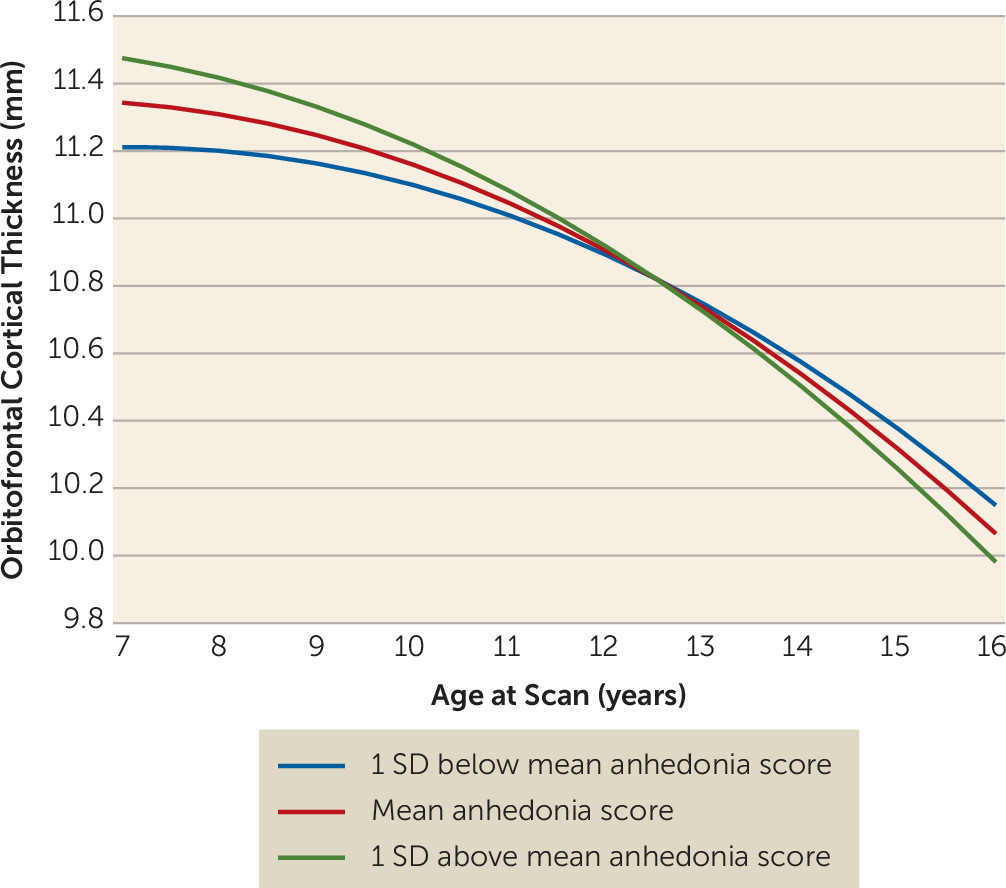
Figure 2). These associations were significant before age 11.37 and after age 15.85. Of note, the interaction between age and depression core score (without anhedonia) was not a significant covariate in either the volume or the thickness model.
Even after accounting for baseline anhedonia, the age-by-anhedonia interaction remained significantly associated with orbitofrontal cortical volume and thickness (see Table S1 in the data supplement that accompanies the online edition of this article). Furthermore, the findings also remained significant after inclusion of depression core score (without anhedonia).
The anhedonia-by-age interaction in the multilevel linear model of striatal volume (residualized for whole brain minus striatum) was nonsignificant (p=0.69).
Trajectories of Orbitofrontal Cortical Volume and Substance Use at Follow-Up
Alcohol use.
As shown in
Table 3, residualized (for race, sex, depression core score, anhedonia score, and age-by-anhedonia interaction) intercepts from the multilevel linear models of orbitofrontal cortical volume and thickness significantly and negatively predicted alcohol use frequency at age 12 (i.e., intercept). Change in alcohol frequency (i.e., slope) was also negatively correlated with orbitofrontal cortical thickness (p=0.006) but not volume (p=0.47). There was no effect on the intercept or slope of the zero-inflated component (i.e., change in volume or thickness did not predict alcohol abstinence or initiation).
Marijuana use.
Residualized orbitofrontal cortical volume and thickness were significantly associated with the slope for the zero-inflated component, i.e., onset of marijuana use, with positive associations for volume (p<0.001) and particularly strong negative effects for thickness (p<0.001) (
Table 4). There was also evidence that change in orbitofrontal cortical thickness (but not volume) was negatively associated with change in frequency of marijuana use (i.e., slope). Intercepts for the zero-inflated component as well as for continuous frequency were unrelated to changes in orbitofrontal cortical structure.
Alcohol or marijuana use.
Alcohol use and marijuana use were moderately correlated with each other (r=0.68). Results of the zero-inflated Poisson models of alcohol or marijuana use are summarized in
Table 5. Residualized (for race, sex, depression core score, anhedonia score, and age-by-anhedonia interaction) intercepts from the multilevel linear models of orbitofrontal cortical volume and thickness significantly and negatively predicted subsequent alcohol/marijuana use frequency (see Figure S1 in the
online data supplement), even after accounting for zero-inflation (i.e., majority nonusers). Thus, even after accounting for prior anhedonia during childhood and its effects on orbitofrontal cortical volume, participants with smaller orbitofrontal cortical volumes at age 12 reported more frequent alcohol/marijuana use 3 years after the scan (p=0.004). The association between the intercept for orbitofrontal cortical thickness and frequency of alcohol/marijuana use was highly significant as well (p<0.001) (
Table 5), even after adjustment for sex, race, and age at alcohol/marijuana use assessment. In addition, slopes from the multilevel linear model of orbitofrontal cortical thickness were significantly (p<0.001) and negatively associated with alcohol/marijuana use abstinence and frequency (i.e., with sharper declines in thickness, the likelihood of onset of alcohol/marijuana use and frequency of use increased).
Supplemental analyses found no association between age at initiation of alcohol/marijuana use and intercepts or slopes for orbitofrontal cortical volume residuals or thickness (see Table S2 in the data supplement). Results for other definitions of alcohol/marijuana use were highly similar to those for the primary phenotype of marijuana use with alcohol use ≥5 drink frequency (see Tables S3 and S4 in the data supplement).
Even after controlling for prior marijuana or alcohol use (i.e., at scans during childhood that were concurrent with orbitofrontal cortex assessment), residualized intercepts for orbitofrontal cortical volume and both intercepts and slopes for orbitofrontal cortical thickness remained associated with alcohol/marijuana use frequency during adolescence (i.e., 3 years after scan 3).
In contrast, depression core score at the later follow-up was not significantly associated with residualized (for race, sex, earlier depression core score, anhedonia score, and age-by-anhedonia interaction) intercepts or slopes from multilevel linear models of orbitofrontal cortical volume (intercept p=0.815, slope p=0.359) or thickness (intercept p=0.411, slope p=0.817), controlling for race, sex, and age.
Anhedonia scores at scans 1 and 2 were not correlated, and anhedonia score at scan 3 was weakly correlated with alcohol/marijuana use frequency (r=0.19, p=0.047). Consistent with this, intercepts and slopes for orbitofrontal cortical volume and thickness extracted from models residualizing for race and sex, but not for anhedonia score or the age-by-anhedonia term, were also significantly and similarly associated with alcohol/marijuana use (see Table S5 in the data supplement). Similar models (without anhedonia) were run for the striatum and were nonsignificant. These findings suggest that while prior anhedonia may play a role in the relationship between the orbitofrontal cortex and later alcohol and marijuana use, some of the relationship between the orbitofrontal cortex and substance use arises independently of anhedonia.
Discussion
Implications for Brain Development
The roles of the orbitofrontal cortex and the striatum in the processing of rewarding experiences have been relatively well established (
4,
39). Deficits in reward processing are known to be associated with risk for substance use disorder and depression in adolescence (
1,
3). The findings presented here, from a longitudinal neuroimaging study of depressed and healthy preschoolers that continued into early adolescence, extend this literature by demonstrating that the developmental trajectory of orbitofrontal cortical volume and level of anhedonia covary together over development during school age and early adolescence. Notably, this effect was driven by thickness of the orbitofrontal cortex and not surface area. This finding suggests that the structure of this region and its reward processing function are linked across childhood development. Of interest, this relationship was not found in the striatum, despite its established role in reward processing. It was also notable that this effect was specific for anhedonia, as this relationship remained even when depression severity without anhedonia was accounted for in the model. Furthermore, it was also specific for the measures of anhedonia proximal to the measures of brain structure, as the findings remained even after controlling for anhedonia measured at baseline during the preschool period. This finding suggests a close dynamic interplay between anhedonia and orbitofrontal cortical thickness across middle childhood development.
The study findings suggest that there is a close link between experiences of anhedonia and orbitofrontal cortical thickness as they change across school-age and early adolescent development. It is notable that this occurs during the developmentally normative process of pruning and myelination and associated gray matter thinning, known to begin in early adolescence (
40). The finding of effects driven by cortical thickness and not surface area is not surprising given that development and variation in cortical thickness and surface area are driven by dissociable genetic (
41–
44), evolutionary (
45), and neurobiological mechanisms (
46–
49) and show different trajectories across neurodevelopment (
50–
56). For example, very early in life, surface area expansion reflects at least in part the generation of cortical columns (
46–
48), while thickness is thought to reflect the creation of neurons within these columns (
46). In healthy children, there is evidence that changes in cortical thickness may be the primary contributor to developmental changes in volume during adolescence (
50), hypothesized to reflect, at least in part, synaptic pruning (
57–
59). Thus, although speculative, it is possible that our findings for orbitofrontal cortical thickness reflect processes related to variation in synaptic modification. However, animal research or human developmental data based on diffusion tensor imaging are needed to test this hypothesis more directly. Interestingly, the finding of both steeper rates of growth and subsequent thinning has been reported in a number of risk conditions and has been dubbed an “acceleration/deceleration” pattern of brain maturation. Such a pattern has been observed in high-risk states (e.g., early-life institutionalization and other forms of extreme stress) described in some mental disorders (
60,
61).
Implications for Substance Use and Risk of Substance Use Disorder
Changes in orbitofrontal cortical volume and thickness were related to frequency of alcohol and marijuana use (both individually and as a composite), and they were not related to later risk for depression. This finding is consistent with the previously reported role of the orbitofrontal cortex in substance use (
14,
15) and suggests that it is specific to substance use disorder rather than depressive outcomes. Because of its role in reinforcement learning and decision making related to valuation of reward, the orbitofrontal cortex has been more notably implicated in the development of cue reactivity and drug seeking in the latter stages of addiction (
14). The orbitofrontal cortex is known to be enriched for CB
1 receptors and is shown to modulate goal-directed behavior (
62). This makes it a specific region of interest for marijuana- and alcohol-related outcomes (
63,
64). Accordingly, atypical orbitofrontal cortical functional connectivity has been noted in heavy marijuana and alcohol users (
13,
16,
35,
65,
66).
While most studies have emphasized orbitofrontal cortical changes as a consequence of prolonged substance use, at least three studies suggest that variability in orbitofrontal cortical volume and thickness may precede substance use. Cheetham et al. (
15) found that orbitofrontal cortical volume at age 12 was associated with onset of marijuana use by age 16. Furthermore, Malone et al. (
25) found that while orbitofrontal cortical volume was negatively related to alcohol use in a dose-dependent manner, these differences were not significant within pairs of twins who differed in their extent of alcohol use, suggesting that genetic and environmental predisposing factors for substance use that twins were matched for also played an important role in the risk relationship. In addition, one study (
67) suggested that prenatal exposure to tobacco smoking may moderate the relationship between orbitofrontal cortical thinning and drug experimentation, again underscoring the importance of considering links between orbitofrontal cortical structure and drug use in addition to causal effects of substance use on the orbitofrontal cortex.
Our finding is also important because it provides a compelling test of an alternative structural brain-based hypothesis for the relationship between substance use and anhedonia or depression in youths (
68). While most studies have proposed self-medication as the primary pathway linking depression/anhedonia and substance use, our longitudinal analysis suggests that anhedonia-related developmental changes in orbitofrontal cortical structure may contribute to future escalations in substance use. Furthermore, the fact that these trajectories did not predict depressive outcomes lends further credence to this developmental neurobiological rather than self-medication model. However, associations between volumetric change in the orbitofrontal cortex and alcohol/marijuana use were also evident in models that did not include anhedonia, indicating that there is also an independent relationship between orbitofrontal cortical volume and substance use in youths. Regardless of whether childhood anhedonia mediates the relationship, our study provides compelling support for the role of dynamic developmental changes in orbitofrontal cortical structure and substance use during adolescence.
Limitations of the study include the availability of only three scan waves. Five or more waves would allow for a more powerful platform for disentangling the direction of effects. However, the availability of three scan waves across adolescence is more than has been commonly available in the literature to date. The use of a sample enriched for depressive symptoms in the preschool period may limit the generalizability of the findings, which suggests that similar investigations should be conducted in community samples. However, the fact that this sample would be at uniquely high risk for depression in adolescence and that orbitofrontal cortical trajectories failed to predict adolescent depression is striking. While key variables were controlled for in this analysis, medication exposure and comorbid disorders were not accounted for. Future studies should investigate neural measures of reward processing during functional MRI in the orbitofrontal cortex and related areas simultaneous to measures of structure across time to further elucidate these relationships. In addition, given the centrality of alterations in reward processing to mental disorders, including depression and substance use disorders, how these change trajectories predict later measures of response to reward in early adulthood would be of critical interest in elucidating the developmental neurobiology of these disorders.