A fundamental unanswered question in the treatment of schizophrenia is that of how long antipsychotic treatment should be continued after the first episode. Many current treatment guidelines recommend that antipsychotics should be used for 1–5 years if relapse does not occur (
1), implying that risk of relapse decreases as a function of time among patients who remain stable. In addition, it has been recommended that because of adverse effects of antipsychotics, long-term exposure should be avoided if possible (
2). The classic study by Robinson et al. (
3) showed that among 104 first-episode patients, discontinuation of antipsychotic treatment was associated with a 4.9-fold higher risk of relapse during 5-year follow-up. Because the vast majority of the study patients relapsed during the first 2 years, it was not possible to assess how long maintenance treatment should last. Eight of 15 patients who relapsed after 2 years of stability had discontinued antipsychotic treatment, which suggested that the duration of treatment should possibly be longer than 2 years. Four randomized controlled trials and one nonrandomized study later observed that 57%−98% of first-episode patients who had been stable for 1–2 years experienced an exacerbation or relapse after discontinuation of antipsychotic treatment (
4–
8); a systematic review therefore concluded that within this time frame, discontinuation cannot be recommended in the absence of health problems due to side effects (
9). A recent randomized controlled trial in first-episode patients found that after 1 year of maintenance treatment, deterioration occurred in 53% of patients after discontinuation of antipsychotic medication (
10). However, because of a lack of long-term studies including sufficient numbers of patients, it is not known whether the relapse risk remains elevated after 2–5 years of stability.
Formulating a more precise estimate for the optimal duration of the initiated antipsychotic treatment would require large patient populations and follow-up periods of 10 years or more. Since it is impossible to conduct randomized controlled trials involving tens of thousands of patient-years, observational studies using large electronic databases are the only realistic way to study this issue. In this study, we assessed the risk of treatment failure (rehospitalization or death) as a function of the duration of antipsychotic treatment prior to discontinuation in a comprehensive nationwide cohort of first-episode patients with schizophrenia.
Method
This study was based on nationwide data drawn from the Finnish population-based registers. Ethical approval was obtained from the Finnish Ministry of Social Affairs and Health.
Study Population
All persons hospitalized for schizophrenia in Finland during the period of 1972–2014 were identified from the Hospital Discharge register, which included all inpatient hospital stays with discharge diagnoses recorded. Schizophrenia was defined as ICD-10 codes F20 or F25 or ICD-9 and ICD-8 codes 295.x. From this cohort, persons with a first hospitalization for schizophrenia during the period of 1996–2014 were identified based on not having been hospitalized for schizophrenia before (N=23,499) and not having used antipsychotic drugs during the year preceding the hospitalization (N=8,719 incident cases) (see Figure S1 in the
data supplement that accompanies the online edition of this article). The primary analysis was restricted to this population of patients who had no previous exposure to antipsychotics in order to avoid survival bias (i.e., to exclude patients who had previously been exposed to antipsychotics and were still alive at the start of follow-up). It has been estimated that over 90% of patients with schizophrenia are hospitalized at least once (
11), and the validity of the schizophrenia diagnosis in this database has been demonstrated previously (
11–
14). Sensitivity analyses without excluding those who used antipsychotics during the year before their first hospitalization for schizophrenia (N=23,499) were conducted. Antipsychotic use (based on Anatomical Therapeutic Chemical classification [
15] code N05A, excluding lithium) was derived from the Prescription register, which includes all reimbursed medication dispensations from pharmacies to all residents since 1995.
After discharge from the first hospitalization, a 30-day definition period was applied to identify users (those who initiated antipsychotic use) and nonusers (those who did not initiate antipsychotic use) during this period. Persons who were rehospitalized during this period (N=1,103) were excluded, as were those who discontinued antipsychotic use or who died, as well as those for whom study follow-up ended (Dec. 31, 2015) during the 30-day period (N=201). Time calculation of use and nonuse began after the 30-day period for 4,217 users and 3,217 nonusers. Those who discontinued use (N=1,714) were defined as users who discontinued antipsychotic use during the follow-up not because of a rehospitalization event, death, or reaching the end of study follow-up. Thus, those discontinuers were not rehospitalized before discontinuation of use. Those who discontinued use were categorized on the basis of time on antipsychotic in outpatient care before discontinuation: 0 years, <1 year, 1–<2 years, 2–<5 years, and ≥5 years. The zero group refers to nonusers, as they were likely using antipsychotics during the hospitalization but not after discharge to outpatient care. For overall analyses comparing antipsychotic use and nonuse, the entire groups of users (N=4,217) and nonusers (N=3,217) were followed up for treatment outcomes.
We used treatment history of clozapine as a proxy marker for treatment resistance, and use of long-acting antipsychotic injection as a proxy marker for poor treatment adherence (at least one filled prescription, yes/no). For each patient who discontinued antipsychotic treatment (after <1, 1–<2, 2–<5, and ≥5 years of use), we matched a user who continued antipsychotic use at this time point, based on time on antipsychotic (by incidence density sampling). Users who continued antipsychotic use were excluded if they discontinued use, were rehospitalized, died, or reached the end of study follow-up before or at this time point. Antipsychotic nonusers were defined at the 30-day definition period as those who did not initiate antipsychotic use during the period. They were followed up until the outcome event (rehospitalization or death), until they started antipsychotic use, or until the end of study follow-up (i.e., they never used antipsychotics during follow-up). Mortality analysis was conducted among the three largest patient groups: nonusers, early discontinuers (within 1 year), and continuous users. For mortality analyses, nonusers were matched with patients who discontinued use after <1 year, similarly to the way users were matched (by incidence density sampling and based on time since the definition period ended, resulting in 1,019 matched nonusers for 1,019 discontinuers). Figure S1 in the online data supplement provides details on the inclusion and exclusion process and the matching procedure, and Figure S2 describes the study design.
Sensitivity analyses without exclusion of prevalent antipsychotic users (i.e., no washout for antipsychotic use) were conducted similarly to those for the main analyses. After excluding patients who were rehospitalized (N=3,221) or who discontinued use or reached end of follow-up (N=599) during the 30-day definition period, 15,220 users and 4,459 nonusers were identified, and 4,366 discontinuers. After matching with users who continued use at the discontinuation event, the numbers of persons in these classes were as follows: <1 year, discontinuers and users, N=2,563, and no match, N=2; 1–<2 years, discontinuers and users, N=710, and no match, N=2; 2–<5 years, discontinuers and users, N=722, and no match, N=4; and ≥5 years, discontinuers and users, N=352, and no match, N=11.
Exposure
Continuous antipsychotic use was modeled with the PRE2DUP method from purchases recorded in the Prescription register (
16–
19). Use of each drug was modeled separately with sliding averages of daily dose and by taking into account stockpiling of drugs, personal purchasing regularity, and possible hospitalizations. Overlapping antipsychotic use periods were combined to retrieve use of “any antipsychotic” drug. During antipsychotic use, patients were considered continuing users if they used multiple antipsychotics at the same time or changed between drugs, as long as they were using at least one antipsychotic drug. Discontinuation was defined as stopping use of antipsychotics, without having an outcome event or end of study follow-up (censored). Nonusers and discontinuers were censored if they started to use an antipsychotic (at least one filled prescription). Thus, the PRE2DUP method was used to define exposure and nonexposure periods for medications (
16–
19). Our previous studies of the validation of the method indicate that PRE2DUP is the most precise method currently available to estimate drug use, and it gives highly accurate drug use periods for most drug classes, especially those meant for long-term use. As variation in dosage is allowed within the method, no artificial grace periods are used.
Outcomes
Because antipsychotics may have both beneficial effects, such as reducing rehospitalizations and suicides, and harmful side effects that may result in increased mortality, the composite outcome “treatment failure,” which included psychiatric rehospitalization or death, was used as a primary outcome measure to evaluate overall net effect related to antipsychotic use. Psychiatric rehospitalization was used as proxy for relapse and was used as a secondary outcome measure, and mortality was analyzed as a secondary outcome measure for the largest patient groups (those who discontinued antipsychotic use within 1 year of use and their matched continuous users and nonusers). Only the first psychiatric rehospitalization was considered for each person.
Covariates
The analyses were adjusted for gender and age at the discontinuation/matching. In sensitivity analyses, the length of the index hospitalization (a proxy for initial illness severity) was also adjusted for, in addition to age and gender. The length of index hospitalization was categorized in quartiles (1–21 days, >21–56 days, >56–116 days, >116 days). Time since the first hospitalization for schizophrenia was taken into account in the matching.
Statistical Analysis
When comparing those who discontinued antipsychotic use with matched users who continued antipsychotic use (and with matched nonusers for mortality), the follow-up time started at the discontinuation/matching date and after the 30-day definition period. Follow-up ended on psychiatric rehospitalization or death (outcome events), end of study follow-up (Dec. 31, 2015), end of antipsychotic use (for users), and restart of antipsychotic use (for nonusers and those who discontinued). Cox proportional hazard models were utilized for comparing risk of treatment failure between discontinuers (0, <1, 1–<2, 2–<5, ≥5 years of antipsychotic use before discontinuation) and matched users (the reference category), with adjustment for age and gender. Secondary analyses with psychiatric rehospitalization as outcome event (censoring for death) were conducted by comparing the risk between discontinuers and matched users, and with death as outcome event by comparing discontinuers and matched nonusers with matched users (the reference category).
In the overall comparison between nonusers and users (without any matching), all nonusers and users as they were categorized during the 30-day definition period were included (3,217 nonusers and 4,217 users). The follow-up started after the definition period and ended on psychiatric rehospitalization or death (outcome events), end of study follow-up (Dec. 31, 2015), end of antipsychotic use (for users), or start of antipsychotic use (for nonusers). Nonusers were compared with antipsychotic users (the reference category) with Cox models adjusted for age and gender.
Incidence rates per 100 person-years (with 95% confidence intervals) were calculated on the basis of the number of events and person-years. Kaplan-Meier survival curves were constructed to visualize time to treatment failure between those who discontinued use and matched users.
Results
The median age of the included cohort (N=4,217 antipsychotic users and N=3,217 nonusers) at cohort entry (on discharge from first psychiatric hospitalization) was 35.0 years (interquartile range, 25.0–51.0), and 56.5% of patients were men. Antipsychotic users were somewhat younger on average (median age, 33.0 years; interquartile range, 24.0–48.0) than nonusers (median age, 38.0 years; interquartile range, 26.0–56.0). At the matching date when the follow-up for treatment outcomes started, the median age of matched users (N=1,714) was 35.0 years (interquartile range, 26.0–49.0) (55.0% were men), and for discontinuers (N=1,714), 34.0 years (interquartile range, 26.0–48.0) (51.4% were men).
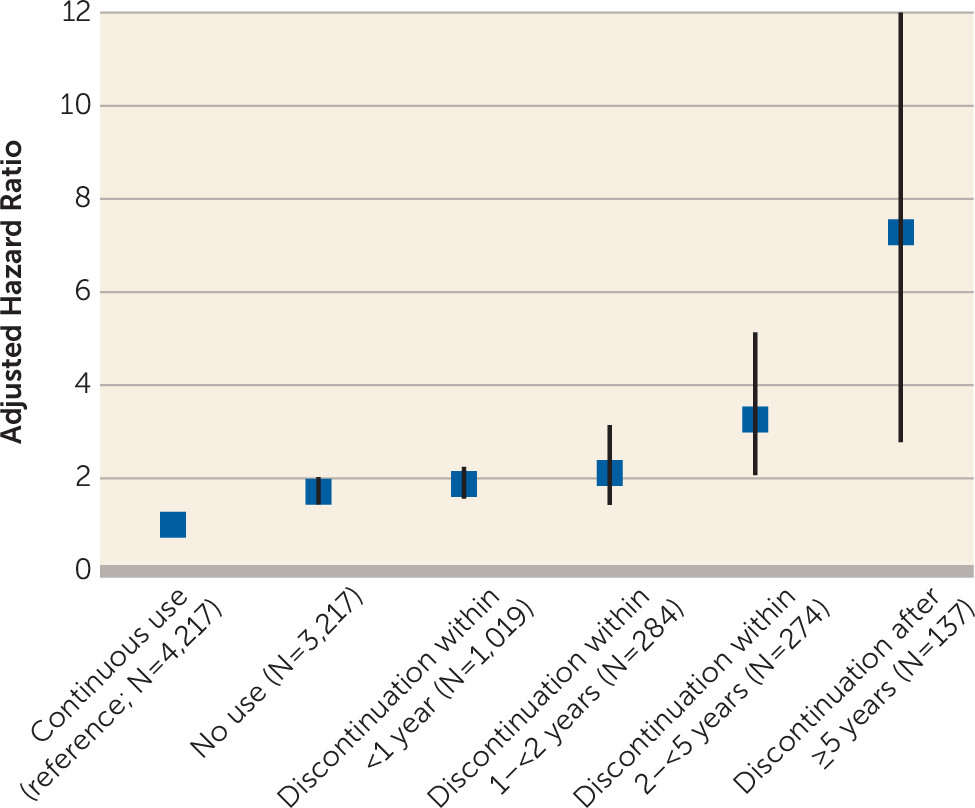
Table 1 summarizes the risk of psychiatric rehospitalization or death after discontinuation of antipsychotic use compared with matched individuals who continued antipsychotic use at each time point. Of discontinuers, 38.0% (N=652) experienced treatment failure, with a median follow-up of 0.5 years (interquartile range, 0.2–1.5), compared with 29.3% (N=502) of their matched users in 1.7 years (interquartile range, 0.6–4.5). In the overall comparison between users (N=4,217) and nonusers (N=3,217), treatment failure occurred in 56.5% (N=1,818) of the nonusers, with a median follow-up of 1.3 years (interquartile range, 0.4–3.9) and in 34.3% (N=1,449) of the users, with a median of 1.1 years (interquartile range, 0.3–3.5). Among the patient group with discontinuation after ≥5 years, the median duration of treatment before discontinuation was 7.9 years (interquartile range, 5.8–10.4).
Figure 1 illustrates the risk of treatment failure as a function of duration of antipsychotic use prior to discontinuation. The risk of treatment failure increases with duration of antipsychotic treatment before discontinuation (p<0.05, Spearman correlation, two-tailed). The results of sensitivity analyses that also adjusted for length of index hospitalization were in line with the primary analysis (
Table 1).
Figure 2 presents the Kaplan-Meier treatment failure survival curves for the patient group who discontinued antipsychotic use and matched comparison subjects who continued antipsychotic use. Time zero represents the date of discontinuation and the corresponding matching date for users (i.e., <1, 1–<2, 2–<5, ≥5 years after discharge from first hospitalization). These figures show that the risk of treatment failure was more pronounced after discontinuation for those with a longer continuous history of antipsychotic use. The corresponding data without exclusion of patients who used antipsychotics during the year before their index hospitalization are presented in Figure S3 and Table S1 in the
online data supplement. The results for secondary analyses on rehospitalization outcome for discontinuation groups are summarized in Table S2 in the
data supplement and in
Figure 3, and corresponding analyses for larger patient populations without exclusion for prior antipsychotic use are summarized in Figure S4 and Table S3 in the
data supplement. The proportions of clozapine and long-acting antipsychotic injection use among discontinuers and matched users are listed in Table S4 in the
data supplement.
The risk of death was compared between those who discontinued within less than 1 year of use and their matched continuous users and nonusers (
Figure 4). When compared with the continuous users, nonusers had a 214% higher risk of death (hazard ratio=3.14, 95% CI=1.29–7.68), and early discontinuers had a 174% higher risk of death (hazard ratio=2.74, 95% CI=1.09–6.89) (see Table S5 in the
data supplement). The gap between users and nonusers and early discontinuers increased progressively as a function of time through 6,000 days (16.4 years).
Discussion
To our knowledge, this is the first study to show how the risk of schizophrenia relapse is modified by the duration of antipsychotic treatment among first-episode patients. The results from this comprehensive nationwide cohort with 20-year follow-up show that the later the antipsychotic is discontinued, the greater the relative risk of treatment failure. This indicates that if antipsychotic treatment is started, no safe time point for discontinuation can be defined, at least during the first 8 years after the first episode. Antipsychotics have beneficial effects in reducing relapses (
20) but also adverse effects, such as cardiometabolic effects (
21–
23), that may lead to increased mortality (
24). Therefore, given that our aim was to evaluate the net effect of antipsychotic use, the primary outcome measure included both psychiatric rehospitalizations and death. While the incidence rate of treatment failure and rehospitalization decreased as function of duration of the preceding antipsychotic use among patients who continued to use antipsychotics, this was not the case among those who discontinued treatment (see
Table 1). One putative explanation for this unexpected finding is that long-term antipsychotic exposure modifies the homeostasis of the brain, which makes discontinuation more difficult when exposure has been longer. This argument is in line with the hypothesized development of supersensitivity of D
2 receptors during long-term antipsychotic treatment (
2). The results remained essentially the same in the sensitivity analysis in which the length of the index hospitalization (a proxy for initial severity of illness) was also adjusted for, but it is possible that this measure cannot describe the severity of illness during the entire follow-up. Since the difference in relapse rates between discontinuers and continuous users increased over several years after the moment of discontinuation, it is obvious that the findings are not largely attributable to supersensitivity of D
2 receptors. As can be seen in
Figures 2 and
3, only a tiny proportion of the relapses occurred during the first 6 months after discontinuation. Since relapses due to supersensitivity should appear abruptly after discontinuation, it is probable that other factors play a much more important role. This interpretation is in line with meta-analyses comparing relapse rates among patients treated with antipsychotics and patients receiving placebo (
20,
25,
26) showing no difference in relapse rates between abrupt and slow withdrawal. Our results are also consistent with the previous finding (
20) that patients randomly assigned to receive placebo after having been stable on antipsychotics up to 6 years had a higher relapse risk compared with those who continued on antipsychotics.
Rehospitalization was a proxy marker for relapse in our study, and we did not have any further information on patients’ coping ability or level of functioning. In the study by Wunderink et al. (
27), level of functioning was better among patients who had early dosage reductions or discontinuations compared with ordinary maintenance treatment, although no difference was observed in the relapse rate. However, it should be noted that the inclusion criterion in that study was not first-episode schizophrenia but first-episode psychosis, and that the majority of patients in the dosage reduction/discontinuation arm were still using an antipsychotic drug without substantial variation in the dosage, and patients on ordinary maintenance treatment had much greater fluctuations in their antipsychotic dosages. The results reported by Wunderink et al. (
27) on early dosage modification are in line with our findings, suggesting that it is safer to modify the dosage in the early phase of the illness, rather than later. A previous study (
28) showed that when thioridazine was withdrawn from the market, the rehospitalization risk doubled after the patients were forced to switch to another medication, which suggests that any change in antipsychotic among stable patients after long-term exposure may be hazardous.
The strength of this study was nationwide coverage of first-episode schizophrenia patients and their follow-up based on register data, which enabled us to obtain information on every individual. The follow-up time in our study was longer than in previous studies that have assessed discontinuation of antipsychotic use. The mean ages at discontinuation in our cohort were rather high but well in line with other first-episode cohorts in Nordic countries (
29,
30). This finding may be partly explained by a delay in setting the final diagnosis, which is suggested by our finding that a large number of patients received antipsychotic treatment before they received a schizophrenia diagnosis. Although previous hospitalization with a schizophrenia diagnosis during the period of 1972–1996 and use of an antipsychotic during the 1-year period before the first schizophrenia diagnosis since 1996 were exclusion criteria, it is probable that some patients had used antipsychotics further back in their history. The risk increase for treatment failure was less pronounced among late discontinuers (after 5 years) in the larger data set, which included patients who had been treated with antipsychotics before their index hospitalization. However, the confidence intervals in this patient group were especially wide, showing a large overlap in the incident cohort and the total cohort. This larger cohort probably also included many chronic patients who were first hospitalized before 1972, when the nationwide database was started. Compared groups (those who discontinued and those who continued antipsychotic use) were matched on the basis of time since first hospitalization for schizophrenia, and thus the duration of illness was controlled for. We used the use of clozapine as a proxy for treatment resistance and the use of long-acting antipsychotic injection as a proxy for poor treatment adherence. These indicators suggested that the late discontinuers had a higher rate of treatment resistance compared with early discontinuers, while no signal was observed for poorer treatment adherence among late compared with early discontinuers. Therefore, the results suggest that the higher risk of relapse among late compared with early discontinuers may be attributable to more severe illness. Also, it should be noted that clozapine use was substantially more common among those who used antipsychotics continuously up to 16.4 years when compared with patients with no antipsychotic use or early discontinuation (23%, 0%, and 9%, respectively), and yet the risk of death remained more than 60% lower among the continuous users than the others. Since clozapine’s adverse effects, including metabolic syndrome, develop over a long period and the accurate cumulative exposure time for specific antipsychotics was not available in this analysis, the outcomes of treatment failure were not adjusted by using this rather crude proxy (at least one filled prescription of clozapine) as a covariate. However, we want to emphasize that the aim of this study was not to investigate clinical characteristics associated with treatment failure but simply to reveal how the risk of rehospitalization or death evolves after discontinuation of antipsychotic treatment in an entire nationwide cohort of first-episode patients.
The majority of the patients who discontinued their medication did so during the first year, which is in line with previous studies in adolescents and adults (
31,
32). We expected to observe that relapse risk decreases as function of time, and if the decrease reached a plateau at a certain time point, that might help in estimating an optimal duration of antipsychotic treatment among stabilized patients. To our surprise, however, the risk of relapse and treatment failure related to antipsychotic discontinuation increased at least through the first 8 years, and no hint of any safe timing for discontinuation of treatment could be observed. This is in line with the finding by Leucht et al. (
20) showing that when patients who have been stable for 3–6 years are randomly assigned to stay on antipsychotic treatment or switch to placebo, those in the placebo group had a higher relapse rate, which was not influenced by the duration of preceding stability.
A limitation of this study is that we cannot be sure whether patients actually used the medication that they obtained from the pharmacy. It is unlikely, however, that substantial proportion of patients would purchase medication repeatedly for long periods without using it. We also lacked data on whether the discontinuation of antipsychotic use was suggested or guided by the treating physician or whether it was the patient’s own decision to discontinue. It is possible that patients who are becoming psychotic might lose their insight and stop taking antipsychotic medication. However, our results show that the deviation in relapse rate between discontinuers and continuers happens mostly between 0.5 and 3 years after discontinuation, and only a small proportion of patients relapsed within the first 6 months after discontinuing medication. This indicates that reverse causality (i.e., relapse leading to discontinuation of medication) does not explain the increased risk of relapse after discontinuation of antipsychotics. Discontinuation of treatment also may result in lack of any contact with and use of health care services, leading to higher morbidity and mortality. About 30% of the early discontinuers were not rehospitalized. However, it is not possible to evaluate their well-being or functional capacity, since not all relapses result in rehospitalization.
Our results suggest that the high risk of treatment failure among late discontinuers may be explained by illness severity and that D2 receptor supersensitivity does not have a major role. Whatever the mechanism is, the practical conclusion for clinicians is the same: if antipsychotic treatment has been used continuously for several years, it is risky to discontinue the treatment. It may be argued that it would be useful to be able to find within this population a subgroup of patients who could discontinue medication without relapsing, in order to avoid the severe potential adverse effects of long-term antipsychotic treatment, which may eventually lead to premature death. However, our results indicate that mortality was substantially lower among those patients diagnosed with schizophrenia who used antipsychotics continuously for up to 16.4 years compared with those who discontinued or never started antipsychotics after their first hospitalization. This suggests that, in general, there is no valid argument for stopping antipsychotic treatment in patients with a first episode of schizophrenia on the basis of concerns about their long-term physical well-being. In any case, regardless of the underlying mechanisms, the results reveal what actually happens in real life in a nationwide unselected patient population receiving treatment for schizophrenia, and they provide definite evidence that, contrary to general belief, relapse risk does not decrease as function of time during the first 8 years of illness. In addition, the results indicate that continuous use of antipsychotics for up to 20 years is associated with lower mortality than no use or early discontinuation. Altogether, the findings suggest that at the group level, long-lasting continuous antipsychotic treatment is beneficial for the majority of patients with first-episode schizophrenia.