Borderline personality disorder is a severe mental disorder that is characterized by sudden distressing changes in mood, unstable relationships, and impulsivity (
1,
2). Levels of substance misuse, deliberate self-harm, and suicide are high among people with borderline personality disorder. The condition occurs globally, with a lifetime community prevalence of over 5% (
3). Although no medications have been formally approved for the treatment of borderline personality disorder, people with this condition receive prescriptions for large amounts of medication (
4), with as many as 90% receiving psychiatric drugs and two-thirds taking long-term antipsychotic drugs (
5–
7).
Rapid changes in mood are one of the hallmarks of borderline personality disorder (
8). This has led to interest in the possibility that mood stabilizers, which improve the mental health of people with bipolar disorder, could also help those with borderline personality disorder (
9). Current practice guidelines on the treatment of borderline personality disorder advocate the use of mood stabilizers for the treatment of impulsive aggression and self-harming behaviors (
10,
11). A systematic review of pharmacotherapy for people with borderline personality disorder concluded that mood stabilizers may be effective in reducing core symptoms of the condition (
12), but trials to date have been small and have not examined long-term effects (
9).
Lamotrigine is an anticonvulsant that has been approved for the treatment of bipolar affective disorder. It is relatively safe in overdose and less teratogenic than some other mood stabilizers (
13–
15). Evidence that lamotrigine may prevent relapse in rapid-cycling bipolar disorder (
16) makes it particularly worthy of testing among people with borderline personality disorder. Two small randomized trials reported reduced impulsivity and affective lability among patients treated with lamotrigine compared with those treated with an inactive placebo (
17,
18). Both were preliminary studies, however, and they examined only short-term effects.
We investigated the clinical effectiveness and cost-effectiveness of lamotrigine for adults with borderline personality disorder who were using secondary care mental health services. We followed people up 52 weeks after randomization to examine the long-term effects of this treatment.
Method
The LABILE (Lamotrigine and Borderline Personality Disorder: Investigating Long-Term Effects) trial was a two-arm, parallel-group, blinded, randomized trial of lamotrigine compared with placebo for adults with borderline personality disorder. Full details of the trial protocol have been published elsewhere (
19). We recruited people age 18 or over who were in contact with mental health services in the United Kingdom. To take part in the study, potential participants had to meet DSM-IV criteria for borderline personality disorder, as assessed by the Structured Clinical Interview for DSM-IV Axis II Personality Disorders (
20). Potential participants were excluded if they met diagnostic criteria for bipolar affective disorder (type I or II), as assessed using the Structured Clinical Interview for Axis I Disorders, or who had a psychotic disorder (
21), were taking a mood stabilizer currently or within the past 4 weeks, had a history of liver or kidney impairment, or had cognitive or language difficulties that prevented them from providing informed consent. We also excluded any premenopausal women who were breastfeeding or pregnant at the time of the baseline assessment, were contemplating becoming pregnant during the following 12 months, or were sexually active and unwilling to take regular contraception. The study was approved by the London Central Research Ethics Committee and the Research and Development departments of the participating provider organizations. The Medicines and Healthcare Products Regulatory Agency gave clinical trial authorization.
Randomization and Blinding
After consenting to participate, eligible patients were asked to complete the Hypomanic Checklist (
22), a short screening questionnaire that can distinguish bipolar disorder from unipolar depression, and the International Personality Disorder Examination screening questionnaire (
23). Local research staff accessed an automated randomization service operated by the Nottingham Clinical Trials Unit that randomly allocated participants in a 1:1 ratio to receive either lamotrigine or placebo. Allocation employed random permuted blocks of varying size, stratified by study center, severity of personality disorder (using data from the International Personality Disorder Examination and criteria developed by Tyrer and Johnson [
24]), and extent of bipolarity (using a cutoff score of 14 on the Hypomania Checklist) (
22).
The randomization system generated a unique code for each participant, corresponding to a predetermined active or placebo allocation. Site pharmacies were unblinded to allocation, allowing selection of trial medication from the appropriate arm. Bottles were blinded at the point of dispensing by removal of a tear-off label that contained a code identifying the contents as lamotrigine or placebo.
All patients, carers, and referring psychiatrists were blind to treatment assignment until 52 weeks postrandomization, except in instances where there was an overdose, pregnancy, or other adverse event that required disclosure. Researchers collecting follow-up data remained blind to treatment assignment in these circumstances. Blinding of researchers, the trial manager, and the trial statistician was maintained until all data entry and processing were completed and the database had been locked. All study researchers, aside from the trial statistician and health economist, remained blind to allocation status until after an initial discussion of trial findings had been completed.
Intervention
All study participants continued to receive usual treatment, which included contact with primary and secondary health services, including access to psychological treatment services and inpatient admission if required. No restrictions were imposed on the use of other treatments, except that they could not receive any additional prescriptions for lamotrigine or any other antiepileptic mood stabilizer. Participants in the active arm of the trial received up to 200 mg/day of generic lamotrigine titrated over a 6-week period depending on how well it was tolerated and clinical response. Treatment dosage was titrated according to the established British National Formulary protocol (
25) but standardized to 14-day intervals. The starting dosage was 25 mg/day. Depending on response and tolerance, this was increased to 50 mg/day after 2 weeks, 100 mg after 4 weeks, and 200 mg thereafter. In keeping with recommendations, the maintenance dosage for women taking the combined oral contraceptive pill was increased to 400 mg daily (
25). Participants in the placebo group received usual treatment plus a prescription for an inert placebo, which was identical in appearance to the active medication but contained lactose monohydrate.
In the light of evidence linking high starting dosages of lamotrigine with adverse skin reactions, we required that participants who had a break in treatment of more than 5 days retitrate medication from 25 mg/day.
Outcomes
The primary outcome measure was symptoms of borderline personality disorder at 12 months as assessed by score on the Zanarini Rating Scale for Borderline Personality Disorder (ZAN-BPD) (
26). The ZAN-BPD is a widely used measure of symptoms and behavioral problems experienced by people with borderline personality disorder. The score ranges from 0 to 36, with higher scores indicating poorer mental health. The ZAN-BPD has been used in previous studies of pharmacological and psychological treatments for people with borderline personality disorder, and it is sensitive to change (
27). The lead researcher (R.S.) was trained to use the ZAN-BPD and supervised all other researchers on the project. We examined the degree of agreement between scores on the ZAN-BPD from pairs of researchers who separately rated 27 participants and found them to be highly correlated (intraclass correlation coefficient=0.98, 95% CI=0.95, 0.99).
The secondary outcome measures were ZAN-BPD scores 3, 6, and 12 months after randomization, together with depression, assessed with the Beck Depression Inventory (
28); deliberate self-harm, assessed with the Acts of Deliberate Self-Harm Inventory (
29); social functioning, assessed with the Social Functioning Questionnaire (
30); use of alcohol and other drugs, assessed with the Alcohol, Smoking, and Substance Involvement Screening Test (ASSIST) (
31); and health-related quality of life, assessed with the EQ-5D-3L (
32). When interviewing participants, researchers assessed side effects of trial medication using a standard form designed to cover the possible effects listed in the British National Formulary entry for lamotrigine (
25). Higher scores on all secondary outcome measures indicate poorer health or functioning, aside from the EQ-5D-3L, for which higher scores indicate lower health-related quality of life. Adverse events were also recorded. Use of health and social care resources and costs was assessed using a modified version of the Adult Service Use Schedule (
33). This questionnaire is used to collect detailed data on use of all hospital and community services, including medication. All secondary outcome measures were assessed 3, 6, and 12 months after randomization except alcohol and drug use, which was assessed at baseline and 12 months later.
Adherence
Researchers maintained regular contact with participants throughout the follow-up period during the initial titration phase, inquiring about side effects and adherence every 2 weeks, and then monthly once a maintenance dosage had been reached. Participants were asked if they had missed doses of medication, and a log was made of the dose dispensed on each occasion. We used these logs to record the number of weeks participants reported taking trial medication and the dose of medication they reported taking each week. We defined adherence with medication as the participant taking uninterrupted medication at a dosage of 100 mg/day or more throughout the study period after the initial titration phase had been completed. We supplemented these data by asking participants to complete the four-item Morisky Medication Adherence Scale at 3, 6, and 12 months (
34). This questionnaire provides a valid estimate of adherence with psychotropic medication (
35); scores range from 0 to 4, with higher scores indicating higher adherence.
Statistical Analysis
The sample size calculation and all data analyses were conducted using Stata, versions 13 and 14 (
36). In a previous trial of problem solving therapy, improvements in mental health and reduced use of emergency medical services were seen among participants who had a 3.6-point reduction in ZAN-BPD score. We needed primary outcome data from 214 participants at 52 weeks to have 90% power to detect a minimal clinically relevant difference of 3.0 points (SD=6.75) in ZAN-BPD score using a significance threshold of 0.05, two-sided. To take account of 15% loss to follow-up, we increased the target sample size to 252.
Details of the statistical analyses were recorded in the Statistical Analysis Plan, which was agreed on with the independent Trial Steering Committee prior to completion of data collection, database lock, and unblinding of the study.
The primary analysis was performed according to randomized treatment, regardless of adherence with allocation and without imputation of missing data. The analysis was adjusted by site, baseline ZAN-BPD score, severity of personality disorder (simple or complex), and the extent of bipolarity (score ≥14 or <14 on the Hypomanic Checklist). For secondary analysis of ZAN-BPD scores, the groups were compared using a mixed model for repeated outcome measures adjusted by the same stratification variables used for the primary analysis. We investigated whether any treatment effects were sustained or emerged later by including an interaction term between treatment with lamotrigine and time in the model. In the absence of a time effect, the effectiveness parameter was the average difference in mean ZAN-BPD score over the 52-week period, along with 95% confidence interval and p value. Further sensitivity analyses were conducted to adjust for any variable with marked imbalance at baseline and to investigate the impact of missing data, using multiple imputation.
We investigated the effect of treatment adherence using complier average causal effect estimation methods according to whether the participant had taken trial medication at a dosage of 100 mg/day or more without interruption during the 52 weeks preceding the final follow-up interview.
The primary cost-effectiveness analysis involved comparing incremental differences in total costs and incremental differences in mental health assessed using the ZAN-BPD. In a secondary cost-utility analysis, we compared incremental differences in costs with differences in quality of life measured using quality-adjusted life-years derived from the EQ-5D-3L.
Analyses of secondary outcome measures used methods similar to those in the primary analysis. We used general linear models for continuous outcomes and logistic regression models for binary outcomes, and regression models with bootstrapping for cost data.
For safety data, including adverse events, serious adverse events, and suspected unexpected serious adverse reactions, we used summary statistics, that is, number of adverse events or side effects of different categories and number and proportion of participants reporting at least one adverse event or serious adverse event within each treatment arm.
Results
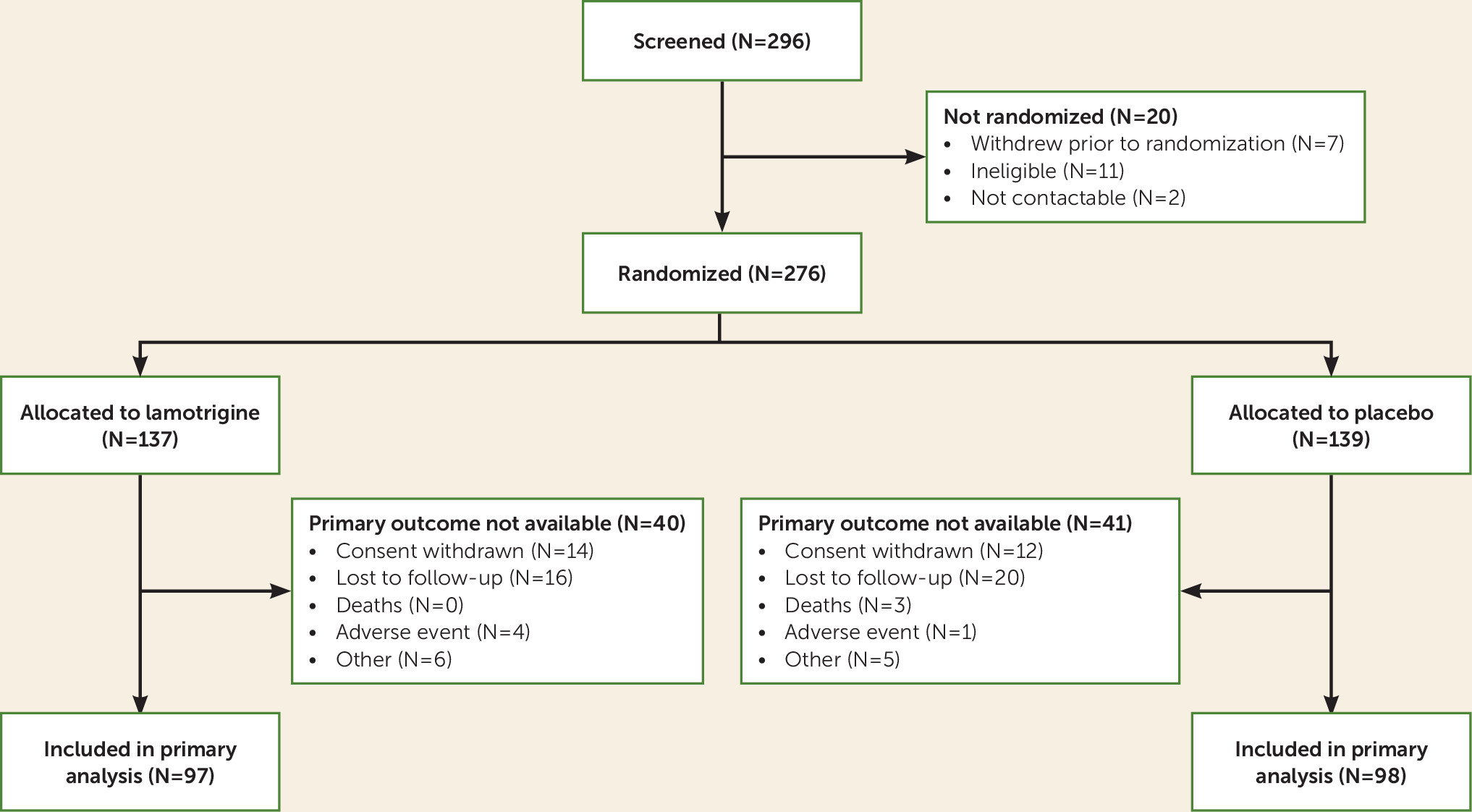
Between July 2013 and October 2015, 296 patients were screened for eligibility. Of these, 276 (93.2%) met eligibility criteria and underwent randomized assignment (
Figure 1), 137 of them to receive lamotrigine plus usual care and 139 to receive placebo plus usual care. There were no instances in which researchers were unblinded to the participants’ allocation status before collection of 52-week outcome data was completed.
The baseline demographic and clinical characteristics of the two study groups were comparable (
Table 1). Follow-up rates were similar between treatment arms, with 195 (71%) participants completing the 52-week follow-up (
Figure 1). In total, 93 (33.7%) participants took trial medication per protocol, and similar proportions were seen in both arms (
Table 2). At 12 weeks, 68.8% were taking trial medication regularly, and 38.9% were taking it regularly at 12 months.
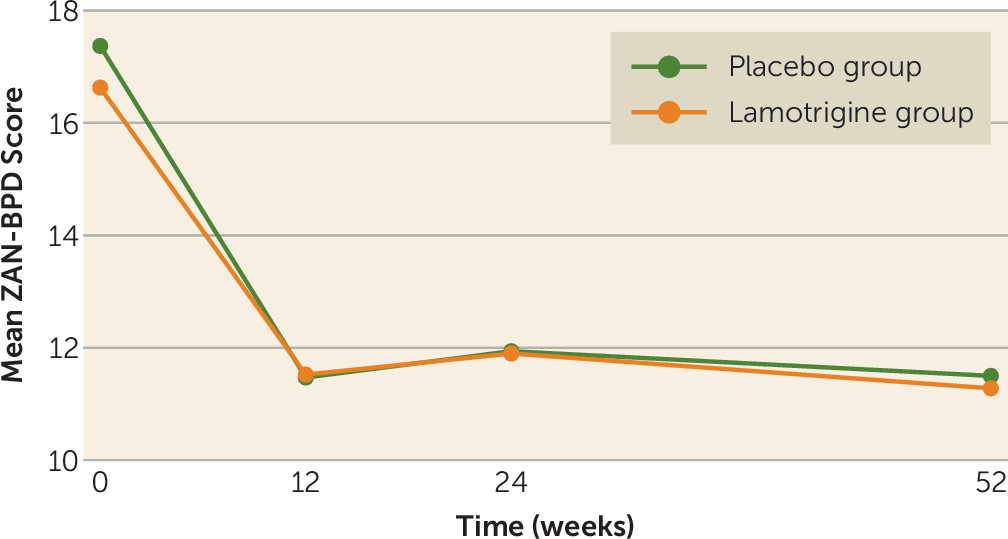
The mean ZAN-BPD score decreased at 12 weeks in both groups, after which it remained stable throughout the remainder of the follow-up. The mean ZAN-BPD scores in the lamotrigine arm of the trial were 11.5 at 12 weeks, 11.9 at 24 weeks, and 11.3 at 52 weeks. Corresponding scores among those in the placebo arm were 11.5, 11.9, and 11.5 (
Figure 2). No difference was found in ZAN-BPD score at 52 weeks between treatment arms. No difference was found in any of the secondary outcome measures or in the four subscores of the ZAN-BPD at any time point (
Table 3; see also Tables S1–S5 in the
data supplement that accompanies the online edition of this article). The lack of treatment effect was supported by sensitivity analyses. The adjusted difference in mean ZAN-BPD score was 0.0 (95% CI=−1.25, 1.26, p=0.90) using repeated measures, −0.1 (95% CI=−1.9, 1.8) using multiple imputation for missing data, and 0.3 (95% CI=−3.7, 4.3) when adjusted for adherence. Regarding adverse events, 77 (56%) of participants in the lamotrigine arm had one or more event, compared with 93 (67%) of those in the placebo arm (
Table 4). The corresponding figures for serious adverse events were 26 (19%) in the lamotrigine arm and 32 (23%) in the placebo arm, including five pregnancies (three in the lamotrigine group and two in the control group).
At baseline, costs were on average $8,160 for participants in the lamotrigine group and $5,163 for those in the placebo group during the 6 months preceding randomization. Average total costs over 52 weeks were $17,785 for the lamotrigine group and $12,340 for the placebo group (
Table 5). The difference in cost was not statistically significant (adjusted difference=$931.99, 95% CI=−2740.44, 4604.41, p=0.62). Group differences between health-related quality of life and the resulting quality-adjusted life-years were also not statistically significant.
Discussion
In this placebo-controlled randomized trial, we found no evidence that prescribing lamotrigine for people with borderline personality disorder led to improvements in their mental health. The study was large enough to generate a precise estimate of the overall treatment effect, which did not include the minimum clinically important difference of 3.0 in ZAN-BPD score at 12 months (the primary outcome measure). Levels of adherence to trial medication were low, with only a third (N=93, 33.7%) of study participants taking trial medication throughout as specified in the study protocol. Levels of adherence were higher during the first 12 weeks of the study, when two-thirds of participants were taking the medication (N=190, 68.8%), but we did not find differences in study outcomes during this period. In a secondary analysis using complier average causal effect methods, we found no evidence that greater adherence to trial medication was associated with any benefit to patients. Most participants reported one or more adverse effects, but those in the lamotrigine arm of the trial were no more likely to report potential side effects than those in the placebo arm.
Strengths and Limitations of the Study
The LABILE trial is the first phase 3 trial of a mood stabilizer for people with borderline personality disorder. One of the main strengths of the study is that we followed participants over a 12-month period. Borderline personality disorder is a long-term condition, but previous drug trials have not examined long-term outcomes (
12). We recruited 11% more participants than we originally planned, and the study size allowed enough precision to exclude a minimum clinically significant difference in the severity of symptoms of borderline personality disorder.
In this pragmatic trial, we attempted to replicate clinical practice. However, one area where we were unable to do this was in the means by which participants obtained their medication. Rather than collecting medication from a local pharmacy, most participants had medication delivered to them in person or by post. This meant that participants had more regular contact with staff than they would have in normal clinical practice. Although levels of adherence to medication were low in this trial, we believe that the additional contact that participants had with researchers meant that adherence may have been higher than would be seen in routine clinical practice.
Comparison With Results of Previous Trials
In contrast to the results of the LABILE study, the two previous randomized trials of lamotrigine for people with borderline personality disorder both reported positive effects (
17,
18). Both trials were smaller, had a larger number of exclusion criteria, and followed participants for shorter periods. Several factors may explain differences between the results of the LABILE study and the two previous studies. Randomization does not guarantee that treatment arms are balanced in small trials, and it is possible that differences in study outcomes in these previous trials resulted from differences in baseline characteristics of the samples. Second, in this pragmatic trial we deliberately kept our exclusion criteria to a minimum. This approach meant that we were able to recruit people with the type of complex and severe problems that people with borderline personality disorder who use specialty mental health services generally have. It is possible that lamotrigine reduces symptoms of borderline personality disorder among people who have less complex and severe mental health problems than those we recruited to the study. In the LABILE trial, we had a rigorous process for maintaining blinding through the use of an automated web-based system that allocated study participants. Methods used to maintain blinding in previous trials are unclear (
17).
Implications for Clinicians
Most people with borderline personality disorder who are in contact with mental health services are being treated with psychiatric drugs (
7), and a quarter of them receive prescriptions for mood stabilizers that have not been approved for borderline personality disorder (
5). Use of lamotrigine is specifically recommended in some textbooks on the treatment of people with borderline personality disorder (
11). Clinicians may feel under considerable pressure to prescribe medication for people with borderline personality disorder, especially at times of crisis (
6). In the absence of clear evidence suggesting benefits associated with any type of medication, nonpharmacological approaches should be offered (
2,
9).
Reductions in symptoms of borderline personality disorder during the course of the trial are in keeping with the results of longitudinal studies showing that the mental health of people with this condition improves over time (
37,
38). However, the pattern of improvement among study participants, with reductions in symptoms during the first 12 weeks of the trial, suggests that regression to the mean or general factors such as instillation of hope may have been responsible for this improvement.
In the LABILE trial, we took great care not to recruit women who were pregnant, who wanted to become pregnant, or who were premenopausal, sexually active, and unwilling to take regular contraception. Despite the assurances that participants gave us, five became pregnant during the trial. While lamotrigine has been shown to be relatively safe in pregnancy, this is not true of all mood stabilizers—notably sodium valproate (
39). Warnings have been issued about the off-label use of sodium valproate in women of childbearing age (
40). Despite this, a recent national audit showed that over 10% of women with borderline personality disorder who are in contact with secondary care mental health services in the United Kingdom are currently being treated with sodium valproate (
5). The pregnancies that occurred during the LABILE trial emphasize the importance of avoiding use of unlicensed medications that are potentially teratogenic for women with borderline personality disorder who are of childbearing age.
Conclusions
Based on the results of this trial, we do not recommend that people with borderline personality disorder be treated with lamotrigine. While pharmacological treatment of coexisting mental health conditions is important, we did not find evidence to support the use of lamotrigine for treatment of the core symptoms of borderline personality disorder.
Acknowledgments
The authors thank all those who took part in the study and clinical staff at Central and North West London NHS Foundation Trust, Derbyshire Healthcare NHS Foundation Trust, Nottinghamshire Healthcare NHS Foundation Trust, Oxleas NHS Foundation Trust, Tees, Esk and Weir Valley NHS Foundation Trust, and West London NHS Mental Health Trust for their help with completing the study, especially Ms. Lauren Bryant, Ms. Adele Mason, Mr. John Lawton, Dr. Ben Lucas, Dr. Paul Mallett, Dr. Tom McLaren, Dr. Ayodeji Morah, Dr. Bal Powar, Dr. Paola Rossin, Dr. Zahir Shah, and Ms. Danielle Wilson. The authors also thank the Clinical Research Network for publicizing the study and supporting the recruitment and follow-up of study participants. They would especially like to thank Pat Daly, Anthony Davis, Tara Harvey, Bianca Hinds-Walters, Natalie Marking, Antoinette McNulty, Lorraine O’Connell, Sandra Simpson, Lisa Thompson, and Audrey Williamson for supporting study recruitment and helping with participant follow-up. The authors also thank members of the trial coordinating team at Imperial College London, including Claudia Adele, Amy Claringbold, Zoe Cotton, Kavi Gakhal, Ana Arbeloa del Moral, Lesley O’Connell, and Dilveer Sually, for overseeing the administration of the trial. The authors are grateful to all members of the Trial Steering Committee (Maxine Patel [chair, 2013–2015], Paul Moran [chair, 2015–2016], Delia Bishara, Oliver Dale, Sandra O’Sullivan, and Jenny Trite) and the Data Monitoring and Ethical Committees (Nicol Ferrier [Chair], Tony Johnson, and Steve Pearce) for their support and guidance. The authors are grateful to Sally Strange for advising on study logistics and Jennie Parker for helping in the interpretation and communication of study findings.