Addiction to diverse substances, including alcohol, tobacco, and illicit drugs, is a leading preventable contributor to global disease burden (
1). In 2016, substance use and use disorders accounted for two-thirds of the estimated 64,026 drug overdose fatalities in the United States (
2), and 88,000 fatalities were associated with alcohol-related adverse effects (
3). Although several pharmacological and behavioral treatments for substance use disorders have shown efficacy in controlled clinical trials, there is a need for more effective treatments. Better knowledge, and better measures, of the functional domains underlying substance use disorders could help in assessing severity, modeling heterogeneity, predicting course, targeting treatments, and detecting efficacy and treatment mechanisms.
Building on conceptual frameworks derived from neurobiology, clinical studies, and social psychology (
4,
5), we recently proposed a neuroscience-based framework (the Addictions Neuroclinical Assessment [ANA]) to better understand the heterogeneity of addiction (
6). This framework postulates that three domains are implicated in the development and maintenance of substance use disorders: incentive salience, negative emotionality, and executive function (
7). As reviewed in detail elsewhere (
6), previous studies have shown group differences between addicted and nonaddicted individuals in various assessments, including neuropsychological and neuroimaging, purporting to measure those domains. Additional disruptions in function in those domains have been demonstrated in some individuals at risk for developing addictive disorders, suggesting that compromised function may be a risk factor for, or a consequence of, addiction. One constraint of these previous studies is that they typically evaluated a single domain of function, such as cognitive control or emotion regulation, rather than using a comprehensive framework. An exception to this pattern is the National Consortium on Alcohol and Neurodevelopment in Adolescence study, which evaluated multiple functional domains in adolescents across the spectrum of alcohol use (
37). That study found differences in cognitive, affective, and motor domains related to levels of alcohol consumption. These initial findings suggest that further testing of a comprehensive model in clinical samples is warranted. The present study attempts to begin such a line of inquiry.
Discussion
In a large and diverse clinical sample enriched for alcohol use disorder, factors representing three neurofunctional domains, incentive salience, negative emotionality, and executive function, underpinned and incorporated results from a broad range of scales and neuropsychological tests. Our findings support the existence of neurofunctional domains relevant to substance use disorders that have been previously theorized (
6,
16) and for which previous evidence in model organisms and humans has focused attention on particular aspects of cognition, behavior, emotion, and circuit function (e.g.,
7,
17,
18).
Factor analyses of clinical data from substance use disorders are limited by the lack of measures that may directly access the underlying domains, thereby increasing the likelihood, if not ensuring, that such studies return class structures based on severity, onset, exposure, and use. Several clinical trials, including Project MATCH for the treatment of alcohol dependence, have targeted treatments to clinical subgroups or patients with particular clinical features. For example, long-term, reward-blunted patients with alcohol dependence may be less responsive to naltrexone, explaining the heterogeneous efficacy of this drug in clinical trials (
19,
20).
We hypothesized that noninvasive deep phenotyping of individuals with and without alcohol use disorder across multiple cognitive, psychological, and behavioral domains might detect latent domains mediating vulnerability and progression of addiction. We asked whether the latent factors discovered from this analysis would align with three neurofunctional domains identified in studies of patients with substance use disorders that have used assessments such as positron emission tomography and functional MRI in humans and neuropharmacological, neurocircuit, and genetic manipulations in animal models. Notably, these methods are more invasive and costly and are not suitable for clinical practice.
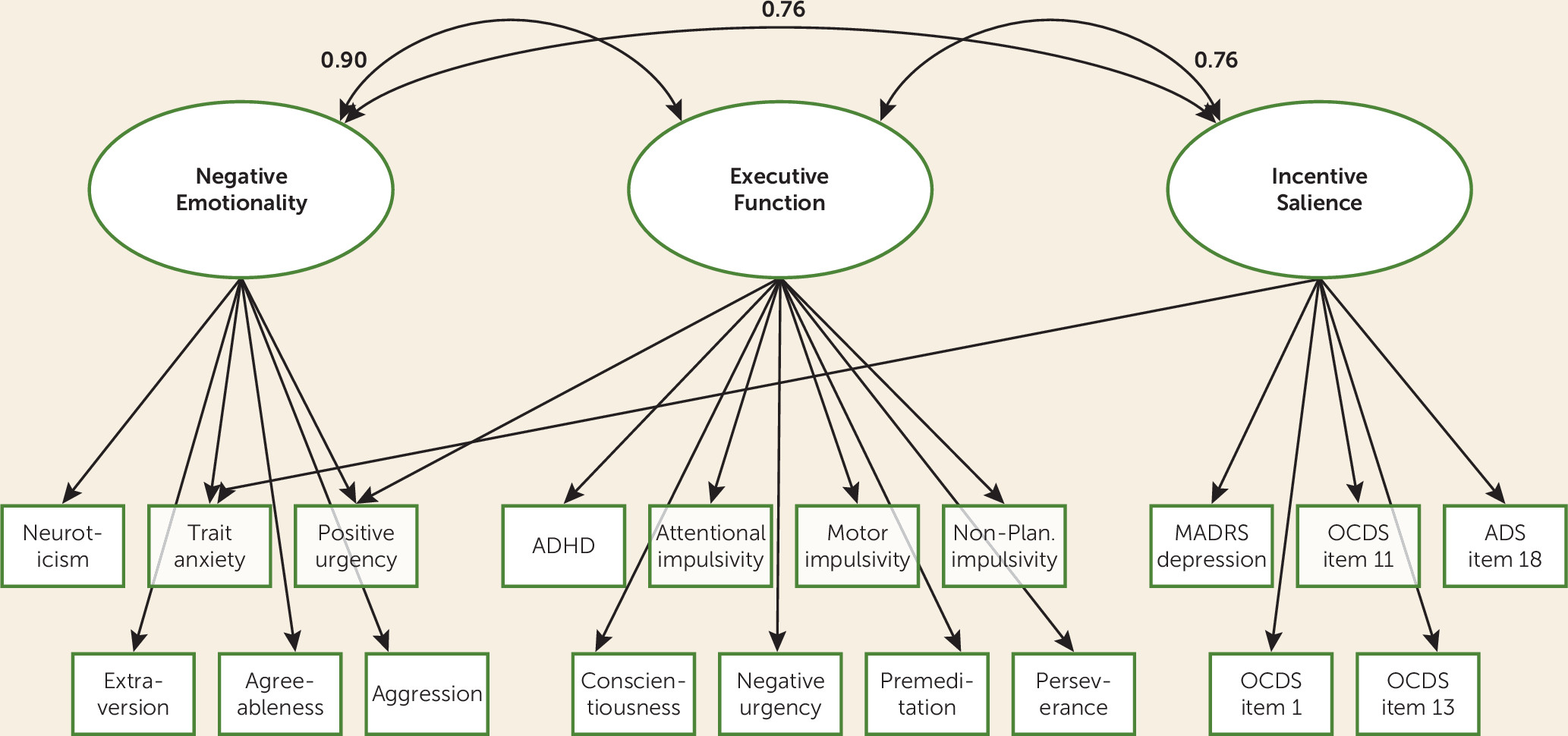
We found that a three-factor model generally demonstrated a good fit with our assessment measures, providing strong support for our first hypothesis. Furthermore, the factors aligned closely with the ANA domains of incentive salience, negative emotionality, and executive function. Relatively inexpensive and noninvasive measures recovered a framework predicted by a large body of preclinical data, clinical psychology, and brain imaging studies (
4,
5). Preclinical data demonstrate decreases in dopaminergic and serotonergic transmission in the nucleus accumbens during withdrawal, paralleled by up-regulation of brain stress systems (e.g., corticotropin-releasing factor, dynorphin) as addiction progresses (
4,
21). This increase in stress during withdrawal is also related to increased vulnerability to relapse and failure to regulate negative affective states in humans. Clinical studies have found that substance use disorders, whether because of innate predisposition or the effect of exposure to various environmental stimuli, such as stress or alcohol, is marked by disruptions of function in prefrontal brain regions implicated in executive function (
22–
24), in striatocortical reward regions implicated in incentive salience (
25), and in the extended amygdala, as implicated in negative emotionality (
26,
27). Neurobehavioral and cognitive assessment could represent a bridge between clinical assessment of symptoms, use, and course and deeper levels of neurofunctional assessment, recovering indices of the same process.
The three principal factors in our sample were intercorrelated, consistent with models of psychopathology based on clinical rather than neuroscience-based assessments (
28). These intercorrelations are likely explained by shared underlying neural circuitry, within and between-system adaptations occurring as part of the addiction process (
29,
30), and shared genetic and environmental risk factors. Increasingly, different substance use disorders demonstrate common disruptions and vulnerability processes. An important goal of deep phenotyping is to define how far sharing of vulnerability and consequences extends. Sharing of vulnerability is observed via family studies and several large, epidemiologically sound twin studies demonstrating cross-inheritance of addictions, as well as agent-specific genetic factors (
31). Clinical research in general population samples has found that remission of one disorder is associated with remission of other disorders and lower probability of new onset of another disorder (
32,
33). Clinical research has also found common outcomes and, for example, higher levels of dysphoria and anxiety in substance use disorder populations, regardless of other subtypology. For example, in one study of patients with substance use disorders from different populations, dysphoria and anxiety were elevated regardless of whether or not patients had antisocial personality disorder features (
34). An important future direction is to examine whether change in any one of the ANA domains is accompanied by change in other domains, in accordance with the relatively strong correlation between domains observed in our sample. If that is the case, treatments that target one domain may have positive spillover effects on other domains. Conversely, it may be essential to simultaneously address several deficits to avoid the possibility that unaddressed deficits may interfere with remission or predispose to relapse.
In the ROC analyses, each of the three neurofunctional domains was moderately to highly accurate in predicting alcohol use disorder, with areas under the curve ranging from 0.84 to 0.96. An important caveat is that a variety of other clinical instruments of different lengths and complexity, but of much greater brevity than the SCID, can also be used to diagnose alcohol use disorder. In one study, the four-item CAGE Questionnaire had an AUC of 0.81 for alcohol dependence in men and 0.75 in women (
35). Our ROC analysis shows that the three neurofunctional domains are germane to alcohol use disorder, but it does not demonstrate that measuring these latent factors can improve clinical assessment of alcohol use disorder or substance use disorders. Nevertheless, the demonstration that the three proposed domains differentiate between participants with and without alcohol use disorder underscores the relevance of these domains for addiction and is a critical finding of this data analysis.
The replication of the three-factor structure in the two split samples provides further support for the robustness of the three-factor solution. Because the ANA model is based on a combination of preclinical and clinical data and had not previously been tested in its entirety, these findings suggest that the development of a battery of assessments designed specifically to measure the ANA domains among individuals with a broader range of substance use disorders is a critical next step for evaluating the validity and clinical utility of this model.
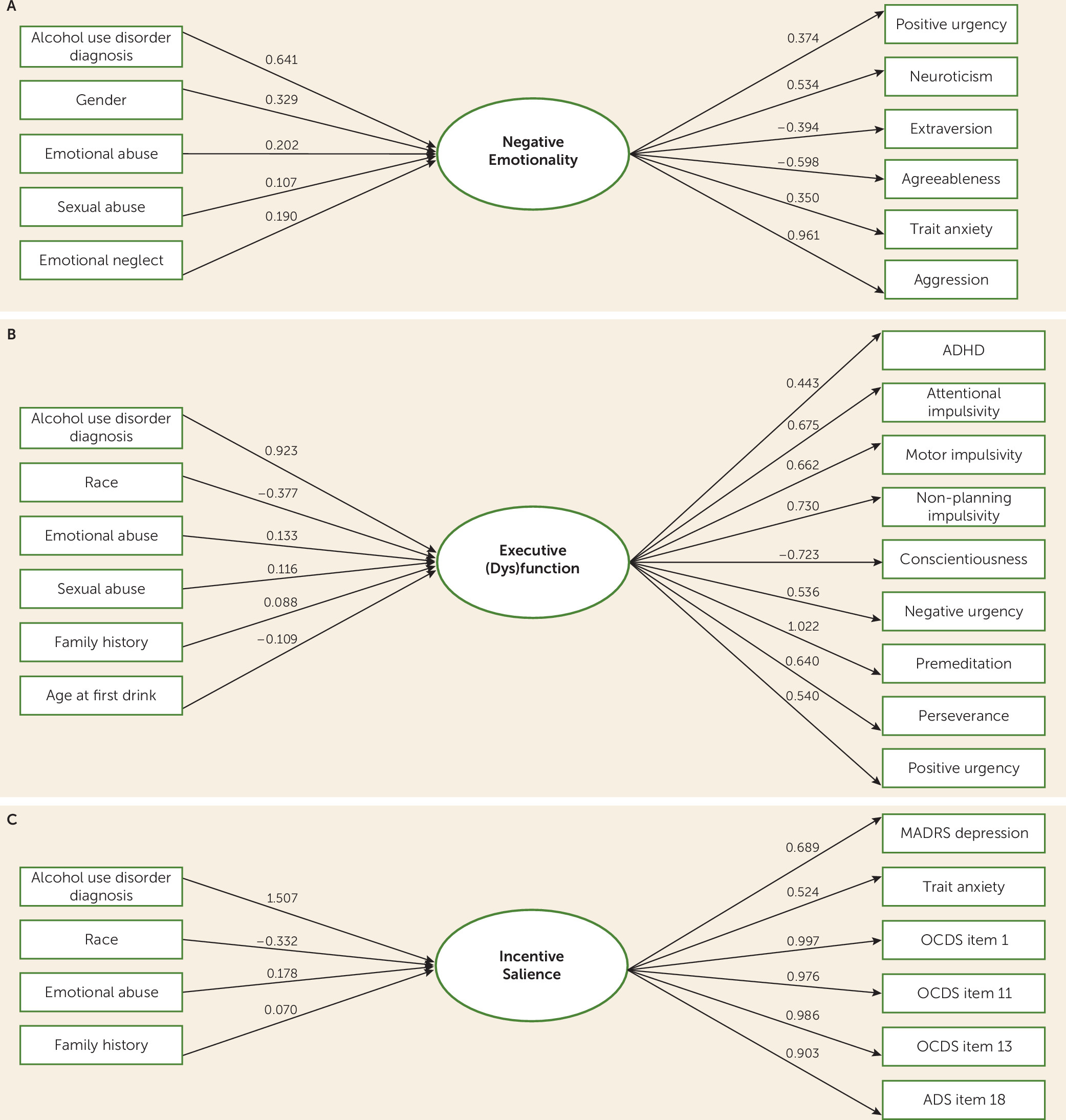
We hypothesized that sociodemographic characteristics would predict severity of the neurofunctional factors. Our MIMIC model indicated that the three domains were associated with genetic (e.g., sex, family history), environmental (childhood adversity), and developmental (age at first drink) correlates. These findings, consistent with biopsychosocial models of substance use disorders (
8–
10), underscore the widespread effects of these risk factors on the neurobiological underpinnings of alcohol use disorder and offer potential targets for prevention. Gene-by-environment interactions may play a role in explaining these findings. For example, individuals with impaired executive function due to genetic predisposition may also be early users of alcohol, which itself is an environmental variable shaping alcohol use disorder onset and progression (
36,
37). In this scenario, an individual may have both a genetic predisposition for impaired executive function and exposure early in life to alcohol as a result of a family environment that may also be influenced by the same genetic liability.
For the negative emotionality domain, it is critical to consider the role of exposure to adverse childhood experiences. Exposure to adverse childhood experiences is one of the strongest risk factors for later development of addictive disorders, likely through genetic and environmental mechanisms (
38). Childhood trauma has an enduring effect on propensity for negative affect (
39) and may lead individuals to consume alcohol and other substances to relieve dysphoria. The MIMIC analysis found that all five subscales of the Childhood Trauma Questionnaire predicted the negative emotionality factor, whereas fewer subscales predicted the other two factors. The finding that Childhood Trauma Questionnaire subscales predicted all three factors constitutes an example of a single predictor leading to disruptions in multiple domains of function, i.e., multifinality (
40).
Finally, although our sample included healthy volunteers as well as individuals with other substance use disorders, we note that differences in factor scores may be secondary to alcohol use disorder—that is, occurring as a consequence of the disease—while others may be preexisting. Many individuals become dysphoric as a function of the development of alcohol use disorder or the use of other substances—the “dark side of addiction” described by Koob and colleagues (
41,
42)—whereas in others, preexisting dysphoria makes them vulnerable to alcohol use and alcohol use disorder (
43). Individuals with alcohol use disorder, regardless of subtype, appear to be more anxious and dysphoric (
44), and in the present study we found strong correlations between the negative emotionality factor and other variables. Increased sensitivity to incentive salience is another correlate of alcohol use disorder. It is also possible that some individuals are predisposed to be less motivated for natural rewards and thus more likely to pursue the exaggerated and immediate reward response to cues and context associated with drugs of abuse. Here, incentive salience is defined as motivation for rewards derived from both one’s physiological state and previously learned associations about a reward cue and is often linked to activation of the mesocorticolimbic dopamine system (
45). Indeed, the fact that depression and trait anxiety scores loaded more heavily onto the incentive salience factor than the (predicted) negative emotionality factor may be considered evidence of a coupling between negative affect and craving for alcohol as addiction becomes severe (e.g.,
7). In this model, craving becomes associated with negative emotions, that is, shifting from “reward” craving to “relief” craving. Finally, the impact of chronic heavy alcohol consumption on executive function may also be profound, while preexisting executive dysfunction is also a known correlate of alcohol use disorder (
46). Thus, across all three domains, chronic alcohol consumption leading to alcohol use disorder has the potential to induce negative emotional states, exaggerated salience for alcohol and related cues, and executive dysfunction.
These results must be interpreted in the context of the study’s strengths and limitations. Strengths include a well-characterized sample of individuals across the spectrum of alcohol use, including individuals seeking inpatient treatment for alcohol use disorder, the use of measures with good psychometric properties, and the fact that the factor structure was replicated across both halves of the randomly split sample. Limitations include that measures were not collected prospectively and may be biased by recollection. Furthermore, although study measures allowed us to recover the three factors hypothesized by the ANA, it is possible that additional factors would emerge if other, more diverse measures were included. We note that measures hypothesized to represent the ANA executive function domain comprise a subset of measures related to cognitive control rather than the broad capacities included in executive function (e.g., working memory, attention, response inhibition, planning), and delay discounting did not load onto this factor.
In summary, the neuroclinical assessment of addictions can capture important dimensions of neuropsychological functioning in individuals with varying levels of alcohol use and use disorders, and these domains are highly relevant to substance use disorders. Future studies combining brain imaging and standardized measures for ANA will help refine our understanding of the relationship of these measures to neural circuits implicated in executive function, negative emotionality, and incentive salience in substance use disorders and other addictive disorders. Further studies will be needed to learn whether assessment of ANA domains can improve prevention or treatment, as already hinted at by treatment studies of substance use disorders.