Alcohol use disorders are modestly heritable, with twin studies demonstrating that approximately 50% of the phenotypic variance is attributed to genetic factors (
1,
2). To date, genetic studies of alcohol use disorders have identified genes that influence pharmacokinetic factors (e.g.,
ADH1B,
ADH1C,
ALDH2) (
3–
8) but none that influence pharmacodynamic factors. The difficulty of assembling large, carefully diagnosed cohorts of individuals with alcohol use disorders has stimulated additional studies of nonclinical phenotypes, such as alcohol consumption, in populations not ascertained for alcohol dependence. This approach has allowed for the relatively rapid collection of much larger sample sizes (e.g., >100,000 individuals) and has identified numerous loci associated with both pharmacokinetic and pharmacodynamic factors that influence alcohol consumption, including
ADH1B/ADH1C/ADH5 (
9–
11),
KLB (encoding β-klotho) (
9,
11,
12), and
GCKR, encoding the glucokinase regulatory protein (
9,
11). However, the genetic overlap between alcohol consumption (in units per week) and diagnosed DSM-IV alcohol dependence is moderate (r
g=0.38) (
13), which reinforces the notion that alcohol consumption cannot be used as a surrogate for alcohol use disorders.
The Alcohol Use Disorders Identification Test (AUDIT) is a screening tool designed to identify past-year hazardous alcohol use (
14). The test consists of 10 items across three dimensions—those pertaining to alcohol consumption (items 1–3, often termed AUDIT-C), dependence symptoms (items 4–6), and problematic or hazardous alcohol use (items 7–10). When the AUDIT was developed, a total score ≥8 was proposed to be indicative of hazardous alcohol use (
14) and a score ≥20 consistent with a diagnosis of alcohol dependence (
15). However, there is no clear consensus on the threshold for alcohol dependence, and subsequent studies have suggested that additional factors, including sex and cultural and social contexts, should be considered when deriving thresholds for alcohol dependence (reviewed in Table S1 in the
online supplement).
In this study, we performed a GWAS meta-analysis using the UK Biobank cohort (N=121,604) and the previously published 23andMe cohort (N=20,328) (
10), yielding the largest GWAS meta-analysis of AUDIT total score to date (N=141,932). Using only the UK Biobank cohort, we also sought to determine whether the alcohol consumption component of the AUDIT had a genetic architecture distinct from the dependence and hazardous-use components by performing GWASs of consumption (items 1–3, AUDIT-C) and problems (items 4–10, AUDIT-P). Linkage disequilibrium score regression (
16) was used to calculate genetic correlations between AUDIT measures and other substance use, psychiatric, and behavioral traits. We also calculated genetic correlations with obesity and blood lipid traits, as these have previously been shown to be associated with alcohol consumption (
9,
10). We hypothesized that AUDIT-P score would correlate more strongly with measures of hazardous substance use, including alcohol dependence, and other psychiatric conditions. Finally, to determine the thresholds for dichotomizing AUDIT total score that would most closely approximate alcohol dependence, we used continuous AUDIT total score to categorize participants as case and control subjects using different thresholds, performed GWAS on each, and calculated the genetic correlation with DSM-IV alcohol dependence (
13).
Results
UK Biobank Sample Characteristics
In the UK Biobank cohort, there were 121,604 individuals with AUDIT scores available for GWAS analysis (see Table S7 in the online supplement). The UK Biobank sample was 56.2% female (N=68,389), and the mean age was 56.1 years (SD=7.7). The mean AUDIT total score was 5.0 (SD=4.18, range=0–40); a histogram showing the distribution of the scores is provided in Figure S2 in the online supplement. Over the previous year, 91.9% of the participants reported drinking 1 or 2 drinks on a single drinking day. Over the previous year, 6.3% of the participants reported that they were not able to stop drinking once they started, and 10.7% felt guilt or remorse after drinking (see Table S7 in the online supplement). The mean AUDIT total score was significantly higher for males than for females (6.09 [SD=4.45] and 4.15 [SD=3.72], respectively; β=0.47, p<2×10−6) (see Figure S3 in the online supplement). In addition, age was negatively correlated with AUDIT scores (β=−0.02, p<2×10−6) (see Table S8 in the online supplement). Therefore, both sex and age were used as covariates in the GWAS analyses. The mean AUDIT-C score was 4.24 (SD=2.83) and the mean AUDIT-P score was 0.75 (SD=2.0). As expected, there was a moderate positive phenotypic correlation between AUDIT-C and AUDIT-P scores (r=0.478, 95% CI=0.473–0.481, p<2×10−16) (see Table S8 in the online supplement).
SNP Heritability in the UK Biobank Sample
We estimated the SNP heritability of AUDIT total score to be 12% (GCTA: SE=0.48%, p=4.6×10
−273; LDSC: 8.6%, SE=0.50%), which is similar to the previously published AUDIT estimate (
10). The SNP heritability for AUDIT-C score was 11% (GCTA: SE=0.47%, p=1.5×10
−211; LDSC: 8.4%, SE=0.55%), and 9% for AUDIT-P score (GCTA: SE=0.46%, p=2.0×10
−178; LDSC: 5.9%, SE=0.48%).
GWAS of AUDIT Scores in the UK Biobank Sample
The significant results (p<5×10−8) of the GWAS of AUDIT total score in the UK Biobank cohort are presented in Table S9 in the online supplement; this analysis revealed 12 independent GWAS signals located in eight loci. The UK Biobank GWASs of AUDIT-C and AUDIT-P score subsets are summarized in Tables S10 and S11 and Figure S4 in the online supplement. Seven of these 12 independent GWAS signals were also significantly associated with AUDIT-C score; the same index variants were identified in the two analyses. An additional GWAS signal was also identified close to FNBP4. For AUDIT-P score, five independent GWAS signals were significantly associated, and these loci were also associated with the total AUDIT and AUDIT-C scores. The rs1229984 SNP in ADH1B was not available for meta-analysis in the 23andMe sample and was not in Hardy-Weinberg equilibrium in the UK Biobank sample used in the present study (p=3.2×10−16); however, in the total UK Biobank white British sample, there was no significant deviation from Hardy-Weinberg equilibrium (p=0.13). The associations between rs1229984 and AUDIT scores are presented in Tables S9, S10, and S11 in the online supplement. The rs1229984 SNP was strongly associated with all AUDIT scores in the UK Biobank sample (β=0.04–0.06, p≤1.0×10−45), but this SNP was not included for clump-based pruning and downstream analyses. We therefore performed a conditional analysis of the SNPs on 4q23 and 4q24 in the UK Biobank sample to determine whether any of these associations were significant after controlling for rs1229984 genotype. While rs13107325 on 4q24 remained significantly associated with AUDIT total score after controlling for rs1229984 genotype, the association between rs146788033, rs11733695, and rs3114045 and AUDIT total score became nonsignificant, suggesting that these loci are tagging the strong rs1229984 signal in this region.
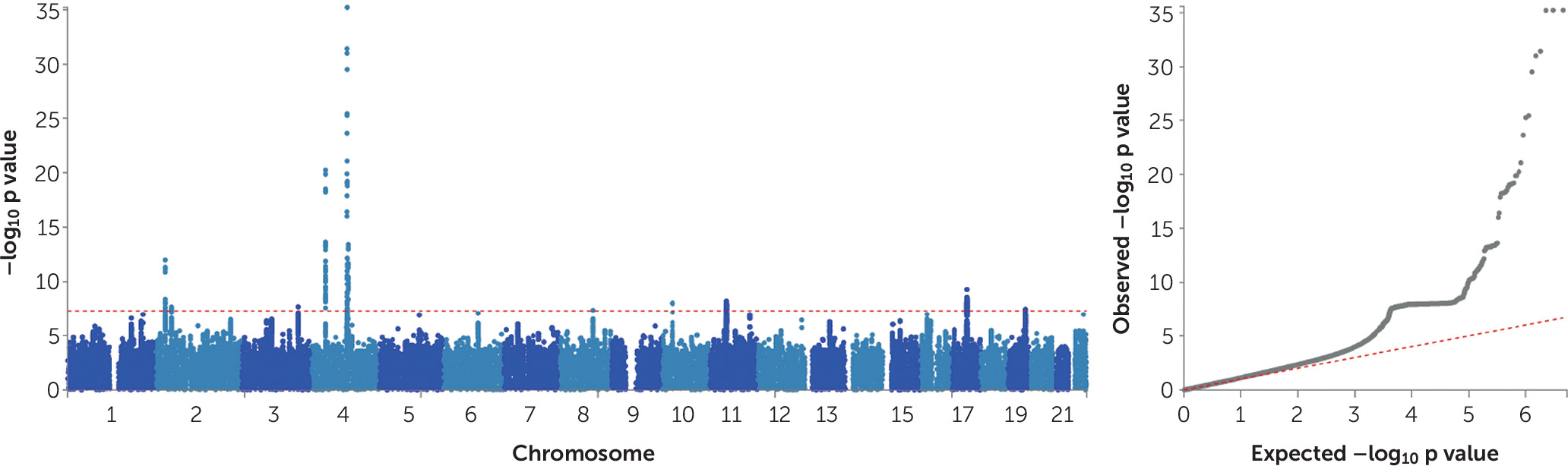
GWAS Meta-Analysis of AUDIT Total Score
The GWAS meta-analysis of the UK Biobank and 23andMe samples found 15 independent GWAS signals (see Table S12 in the
online supplement) associated with AUDIT total score spanning 10 genomic loci (
Table 1).
Figure 1 presents the Manhattan and quantile-quantile plots of the GWAS meta-analysis of AUDIT total score, and Figures S5–S14 in the
online supplement present the regional association plots for the independent signals. The inflation factor of the meta-analysis GWAS was λ
GC=1.22 with an LDSC intercept of 1.008 (SE=0.007), suggesting that the majority of the inflation is due to polygenicity. The 15 independent SNPs show 100% sign concordance for association with AUDIT total score across the UK Biobank and 23andMe samples (
Table 1); 11 of these SNPs were nominally associated with AUDIT total score in the 23andMe sample (p≤0.05), and all index SNPs were associated with AUDIT total score in the UK Biobank sample (p<1.8×10
−6).
The top hit for the GWAS meta-analysis of AUDIT total score was a variant (rs11733695) located downstream (879 base pairs) from
ADH6 (p=3.4×10
−30), which is a member of the alcohol dehydrogenase gene family. The rs11733695 SNP is in low LD (r
2=0.17) with the functional SNP in
ADH1B, rs1229984, which is known to alter alcohol metabolism (
26). In addition, two other regions in 4q23 were associated with AUDIT total score in the meta-analysis: one of the index SNPs was located in the
ADH1C gene; however, conditional analysis of this region in the UK Biobank sample alone suggests that these multiple hits may in fact be tagging the rs1229984 signal. This region has been previously associated with alcohol consumption, alcohol use disorders, and AUDIT scores (
6,
7,
9,
10).
We also replicated the association between
KLB (see Table S12 in the
online supplement), on chromosome 4q14, and alcohol consumption (
9,
11,
12); the index SNP rs11940694, which is located in the intron of
KLB, was associated with AUDIT total score in the present study. Clump-based pruning identified rs11940694 and rs4975012 as independent hits in the
KLB region. Credible set analysis suggests that rs2046330 is the index SNP in the region represented by rs4975012 (see Table S13 in the
online supplement). AUDIT total score was also associated with SNPs that localized to
GCKR on chromosome 2p23.3, which has been previously associated with alcohol consumption (
9,
11). Five SNPs comprised the credible set at the
GCKR locus, which also spans the
SNX17 gene, including the missense variant (rs1260326) in
GCKR that was identified as the index SNP.
We identified GWAS signals in several regions that have not been previously implicated in the genetics of alcohol use disorders, including 2p21, 17q21, 3q25, 8q22, 10p11, 11p11, and 19q13. The index SNP rs13135092 in the 4q24 region is located in an intron of
SLC39A8; the remainder of the credible set for this locus is located in a noncoding pseudogene,
RN7SL728P.
SLC39A8 is highly pleiotropic (
27), but it is a novel association in relation to alcohol. A region of association on 2p21 contains 17 SNPs that are localized to the noncoding RNA
LINC01833. A novel region of association was also detected on chromosome 10p11.23; this region contains nine credible SNPs that localize to the
JCAD (junctional cadherin 5 associated) gene.
JCAD encodes an endothelial cell junction protein and has previously been associated with coronary heart disease (
28).
The remaining novel associations on 3q25, 8q, 11p11, 17q21, and 19q13.3 were more complex. The index variants on chromosome 8q22.1 were not localized to any genes, and it is unclear from the credible set analyses what the causal variants may be at these loci. The credible SNP sets for the 3q25.33, 11p11.2, 17q21.31, and 19q13.3 regions contained more than 50 SNPs each, which spanned several genes. For example, the index SNP on chromosome 17q21.31 was an intronic SNP in
MAPT, which encodes the tau protein and has been robustly associated with Parkinson’s disease (
30,
31) (see Table S14 in the
online supplement) and other neurodegenerative tauopathies (
32) and, more recently, with neuroticism (
33). However, we note that the region of association on chromosome 17q21.31 spans the corticotropin receptor gene (
CRHR1), which has been associated with alcohol use in animals and humans (
34). Thus, because of the extended complex LD in this region, we are unable to determine the likely causal variant. Similarly, the index SNP (rs2293576) at chromosome 11p11.2 is a synonymous SNP of the zinc transporter gene
SLC39A13; however, this region includes 54 associated SNPs, which map to five additional genes. Lastly, the index SNP on 19q13.3 is a synonymous variant in
FUT2.
FUT2 encodes galactoside 2-alpha-
l-fucosyltransferase 2, which controls the expression of ABO blood group antigens. However, 70 SNPs form this credible set, and they span four genes in total.
We used FUMA to functionally annotate all 1,290 SNPs in the credible sets (see Table S13 in the
online supplement). The majority of the SNPs were intronic (76.9%; N=993) or intergenic (11.6%; N=149), and only 26 SNPs (2.0%) were exonic. Furthermore, 38 SNPs showed CADD scores >12.37, which is the suggested threshold to be considered deleterious (
35). The exonic SNPs (rs601338, rs17651549, rs13107325) of
FUT2,
MAPT, and
SLC39A8, respectively, had the highest CADD scores (>34), suggesting potential deleterious protein effects. Overall, 164 SNPs had RegulomeDB scores of 1a−1f, showing evidence of potential regulatory effects; 90.1% of the SNPs were in open chromatin regions (minimum chromatin state, 1–7).
Gene-Based and Pathway Analyses
We used MAGMA (
22) to perform a gene-based association analysis, which identified 40 genes that were significantly associated with AUDIT total score (p<2.70×10
−6) (see Table S15 and Figure S15 in the
online supplement). As expected, the majority of these genes were in the 10 GWAS loci (i.e.,
KLB,
GCKR);
DRD2 and
CRHR1 were also among the top hits. In addition, the analysis revealed a strong burden signal in
CADM2 (p=1.64×10
−9), where the index variant in GWAS meta-analysis did not reach genome-wide significance. We did not identify any canonical pathways that were significantly associated with AUDIT score (see Table S16 in the
online supplement).
Gene-based (MAGMA) analyses for the AUDIT-C and AUDIT-P subsets (see Figures S16 and S17 in the online supplement) revealed evidence of overlap (see Figure S18 and Table S17 in the online supplement). Two genes (KLB, CADM2) were associated with all three AUDIT traits (AUDIT total score, AUDIT-C score, and AUDIT-P score). There was considerable overlap between AUDIT total score and AUDIT-C score, with 21 overlapping genes associated at the gene-based level. Only one gene, DRD2, was associated with both AUDIT total score and AUDIT-P score.
S-PrediXcan
S-PrediXcan identified a positive correlation (p<1.07×10−6) between AUDIT total score and the predicted expression of 26 genes across multiple brain tissues (full results are presented in Table S18 in the online supplement), including MAPT (cerebellum), FUT2 (caudate and nucleus accumbens), and CRHR1-IT1 (putamen, cerebellum, hippocampus, anterior cingulate cortex). SNPs in the region of MAPT and FUT2 were associated with AUDIT total score in the GWAS. MAPT (cerebellum), FUT2 (nucleus accumbens), and CRHR1-IT1 (caudate, cortex, nucleus accumbens, hypothalamus, hippocampus) were also associated with AUDIT-C score (see Table S19 in the online supplement). S-PrediXcan for AUDIT-C and AUDIT-P scores (see Table S20 in the online supplement) revealed that lower predicted RFC1 expression in the cerebellum and hemisphere, respectively, was associated with higher scores on both the AUDIT-C (p=7.84×10−7) and the AUDIT-P (p=1.54×10−6).
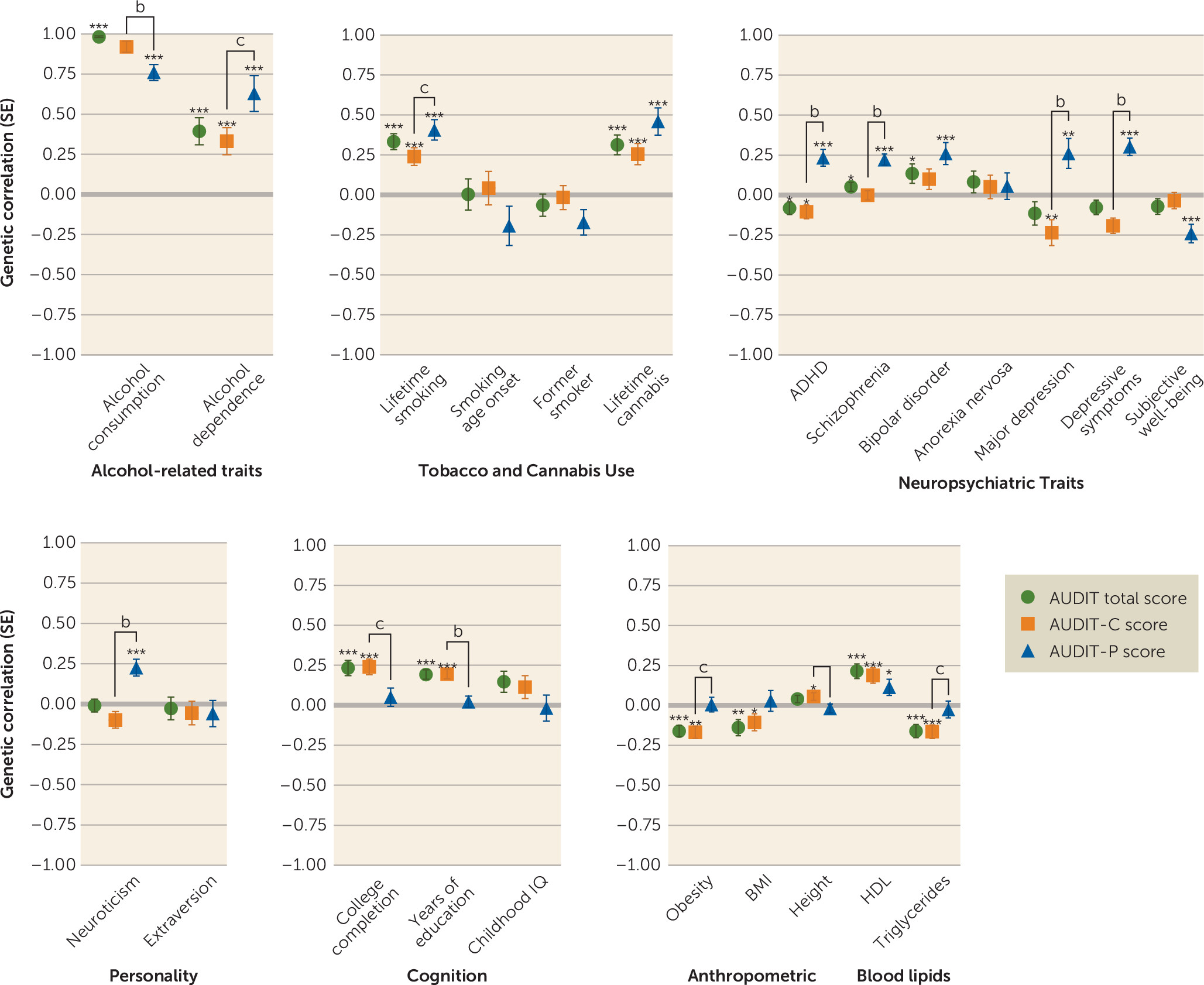
Genetic Correlations
We used LDSC to evaluate evidence for genetic correlations between our three primary traits (AUDIT total score, AUDIT-C score, and AUDIT-P score) and numerous other traits for which GWAS summary statistics were available; these included alcohol and substance use traits, personality and behavioral traits, psychiatric disorders, blood lipid levels, and brain structure volumes (see
Figure 2; see also Tables S3–S5 in the
online supplement).
As expected, AUDIT-C and AUDIT-P scores were highly genetically correlated (rg=0.70, p=4.1×10−70). AUDIT scores (AUDIT total score, AUDIT-C score, and AUDIT-P score) showed strong genetic correlations with alcohol consumption from two other studies (rg=0.76–0.96, p<2.3×10−9). Many of the genetic correlations with AUDIT-P score were significantly different from the correlations with AUDIT-C score (see Table S6 in the online supplement). AUDIT-C score had a significantly stronger (p=8.02×10−3) correlation with alcohol consumption (rg=0.92, p=7.0×10−164) than did AUDIT-P score (rg=0.76, p=2.7×10−52). In contrast, AUDIT total score and AUDIT-C score were only modestly correlated with alcohol dependence (rg=0.39 and 0.33, respectively, p<8.2×10−5), whereas AUDIT-P score showed a nominally stronger genetic correlation with alcohol dependence (rg=0.63, p=1.8×10−8; AUDIT-P compared with AUDIT-C, p=0.033) (see Table S6).
We detected positive genetic correlations between AUDIT scores (AUDIT total, AUDIT-C score, AUDIT-P score) and other substance use phenotypes, including lifetime smoking (rg=0.24–0.41, p<1.6×10−5) and cannabis use (rg=0.26–0.46, p<1.1×10−4). We also observed a positive genetic correlation between AUDIT-P score and cigarettes per day (rg=0.28, p=4.0×10−3).
Several psychiatric disorders and related traits were positively genetically correlated with AUDIT-P score, including schizophrenia (rg=0.22, p=3.0×10−10), bipolar disorder (rg=0.26, p=1.5×10−4), ADHD (rg=0.23, p=1.1×10−5), and major depressive disorder (rg=0.26, p=50.6×10−3). Intriguingly, AUDIT-C score was negatively correlated with major depressive disorder (rg=−0.23, p=3.7×10−3) and ADHD (rg=−0.10, p=1.8×10−2), whereas AUDIT-P score showed positive genetic correlations with these same disease traits (rg=0.26, p=5.6×10−3; rg=0.24, p=1.1×10−5). We observed a positive genetic correlation between AUDIT-P score and depressive symptoms (rg=0.30, p=3.0×10−8) and neuroticism (rg=0.18, p=2.6×10−4) and a negative genetic correlation with subjective well-being (rg=−0.24, p=4.0×10−5).
We observed positive genetic correlations between AUDIT total score and AUDIT-C score and education, college completion, and cognitive ability (rg=0.19–0.24, p<1.5×10−5). The genetic correlations between AUDIT-P and education and college completion were near zero and were significantly lower than AUDIT-C score and AUDIT total score or education traits (see Table S6 in the online supplement).
There were negative genetic correlations between AUDIT total score and AUDIT-C score and obesity (r
g=−0.16–0.17, p<1.1×10
−5), similar to previous reports regarding AUDIT total score (
10) and alcohol consumption (
9). In contrast, obesity was not significantly genetically correlated with AUDIT-P score (r
g=0.01, p=0.90). Similarly, HDL cholesterol and triglyceride levels were genetically correlated with AUDIT total score and AUDIT-C score (r
g=0.19–22, p<9.3×10
−5, r
g=−0.16, p<1.0×10
−4, respectively), but this association was not found for AUDIT-P score (r
g=0.11, p=2.2×10
−2, r
g=−0.03, p=6.4×10
−1). Obesity showed significantly different correlations with both AUDIT-P and AUDIT-C scores (see Table S6).
Dichotomizing AUDIT Total Score to More Closely Approximate DSM-IV Alcohol Dependence
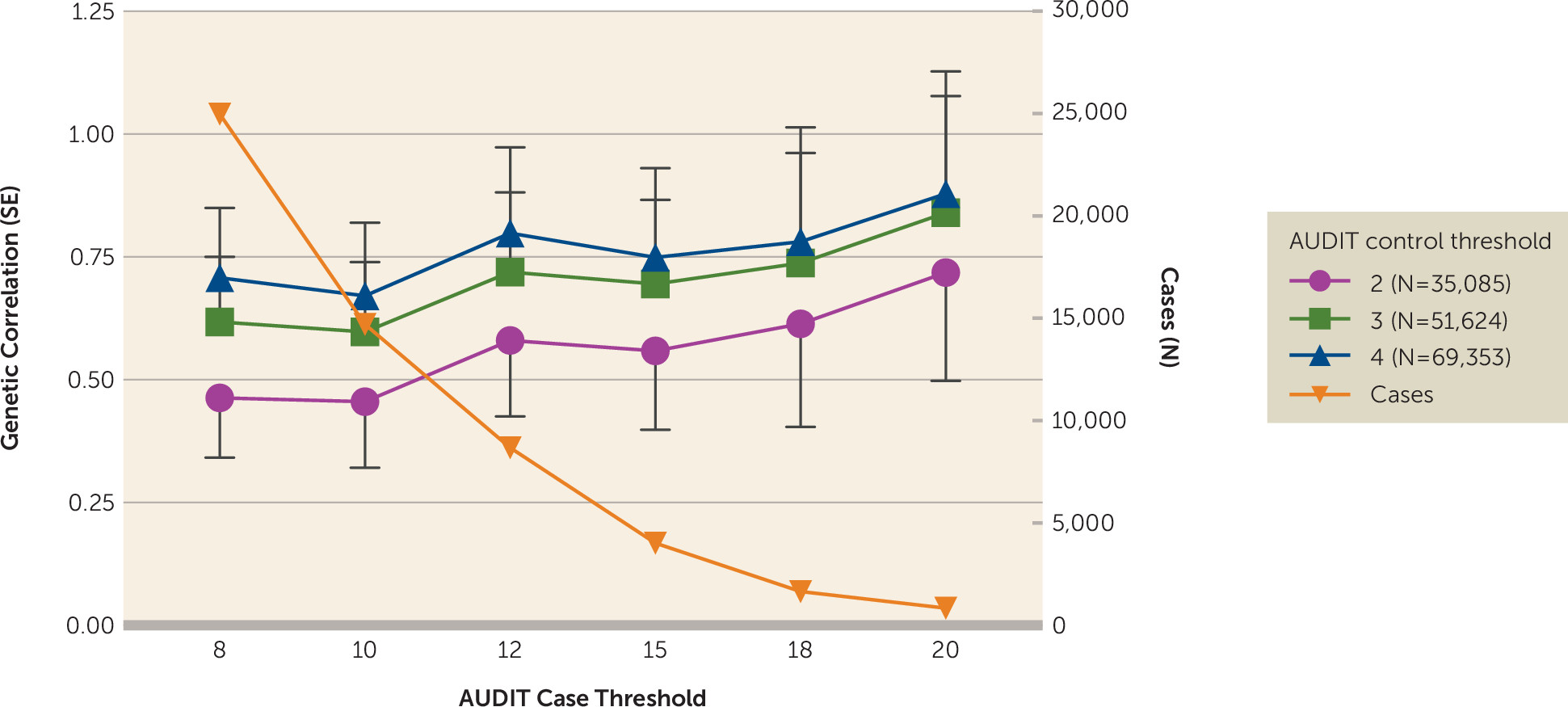
As AUDIT can be rapidly ascertained in large populations, we explored methods for dichotomizing AUDIT total score to optimize the genetic correlation with DSM-IV alcohol dependence (
13). Higher genetic correlations with alcohol dependence were observed as the control threshold was increased from 2 to 4, and with increasingly stringent case cutoffs (
Figure 3; see also Table S2 in the
online supplement). The highest genetic correlation was observed for cases with AUDIT total score ≥20 and controls ≤4 (r
g=0.90, SE=0.25, p=3.0×10
−4); however, this highly stringent threshold produced very few cases (N=1,290). The standard error of the estimate is much larger at more stringent case thresholds, and therefore these estimates should be interpreted with caution. Defining cases as ≥12 yielded an r
g of 0.82 (SE=0.18, p=3.2×10
−6) while retaining more than seven times as many cases (N=9,130); these genetic correlations were not significantly different from those obtained using cases ≥20 and controls ≤4 (p=0.80).
Discussion
We have presented the largest GWAS meta-analysis of AUDIT total score to date, using large population-based cohorts from the UK Biobank and 23andMe. We identified novel associations with AUDIT total score; the genes located in these regions include JCAD and SLC39A8. We found evidence for association in several loci previously associated with alcohol use via single-variant and gene-based analyses (i.e., KLB, GCKR, CADM2). The SNP heritability of all AUDIT phenotypes ranged from 9% to 12%, demonstrating that common genetic factors account for a prominent proportion of the variation in alcohol use phenotypes. Furthermore, we showed that there is shared genetic architecture between AUDIT scores and other alcohol and substance use phenotypes. AUDIT-P score showed a positive genetic correlation with several psychiatric diseases, distinguishing AUDIT-P score from AUDIT-C score. Finally, using LDSC, we identified thresholds for dichotomizing AUDIT total score (score ≥12 to define cases and ≤4 to define controls) that maximize the genetic correlation with alcohol dependence while retaining a large number of participants.
Our top GWAS hits replicated previous association signals for alcohol use traits. The strongest associations with AUDIT score in this study spanned the alcohol metabolism genes on chromosome 4q23 (
10). Variants in this region were associated with AUDIT total score, AUDIT-C score, and AUDIT-P score, demonstrating that alcohol metabolism is a risk factor for both alcohol consumption and problematic use. The second strongest signal, also associated with the three AUDIT phenotypes, is located in
KLB, confirming the robust association of this gene with alcohol consumption in both humans (
9,
11,
12) and mice (
12). However, the biology of this locus could be more complex than previously described. Although the credible set analysis suggested that the more probable causal variants are all located on the first intron of
KLB, one of these variants, rs11940694, is an expression quantitative trait locus for
RFC1 expression in the brain, and
S-PrediXcan analysis predicted that lower expression of
RFC1 in the brain is associated with higher predicted AUDIT (AUDIT-C and AUDIT-P) scores. Interestingly, a gene in the complex GWAS signal on chromosome 19, Fibroblast growth factor 21 (
FGF21), was associated with AUDIT scores (AUDIT total score, AUDIT-C score, AUDIT-P score) at the gene-based level (see Table S17 in the
online supplement).
FGF21 regulates sweet and alcohol preference in mice as part of a receptor complex with β-Klotho (
KLB) in the central nervous system (
33). Additionally, we replicated the association between rs1260326 in the gene
GCKR and alcohol consumption (
9,
11), here associated with AUDIT total score and AUDIT-C score. Other loci previously associated with alcohol consumption include
CADM2 (
9), which was associated at the gene-based level for all three AUDIT traits. Here, the burden analysis suggests that multiple (rare and common) variants are necessary to explain the association signal. Intriguingly, several of the novel associations with AUDIT scores were mapped to highly pleiotropic genes (
MAPT,
FUT2,
SLC39A8) (
27).
Genetic analysis of the AUDIT subsets revealed evidence of distinct genetic architecture between AUDIT-C and AUDIT-P (alcohol consumption versus problem use), with support from the gene-based (see Figures S16 and S17 in the
online supplement),
S-PrediXcan (see Tables S19 and S20 in the
online supplement), and genetic correlation analyses (see
Figure 2). Furthermore, AUDIT-P score showed a strong genetic correlation with alcohol dependence (
13). In contrast, AUDIT-C score had a stronger genetic correlation with alcohol consumption. Thus, partitioning AUDIT scores into different subsets (alcohol consumption versus problem use) may disentangle genetic factors that contribute to different aspects of vulnerability to alcohol use disorders.
Genetic overlap was observed for all measures of AUDIT and other substance use traits, including lifetime tobacco and cannabis use, as we previously reported (
10,
36,
37), demonstrating that genetic risk factors for high AUDIT scores overlap with increased consumption of multiple drug types.
We found several significant differences between the genetic correlations with AUDIT-P and AUDIT-C scores. These differences were particularly pronounced for psychiatric and behavioral traits. AUDIT-P score was positively genetically correlated with psychopathology (schizophrenia, bipolar disorder, major depressive disorder, ADHD), personality traits (including neuroticism), and regional brain volumes. These associations have previously been observed at the phenotypic level; alcohol use disorders commonly co-occur in individuals with schizophrenia (
38), bipolar disorder (
39), major depressive disorder (
40), and adult ADHD (
41). Intriguingly, genetic risk for high AUDIT-C score was
negatively correlated with major depressive disorder and ADHD, demonstrating that a distinct genetic component of AUDIT-P is shared with genetic risk for psychiatric disease. Regional volume abnormalities in subcortical brain regions of individuals with alcohol use disorders have been reported (
42–
44), although it is unclear whether these alterations are a result of high alcohol consumption or a preexisting susceptibility. We identified a positive genetic correlation between AUDIT-P score and increased caudate volume; however, the majority of studies report reductions in regional brain volumes associated with alcohol use disorders.
For AUDIT total score and AUDIT-C score, we showed positive genetic correlations with educational attainment and cognitive ability and negative genetic correlations with obesity, consistent with earlier reports (
9,
10). These associations were not observed for AUDIT-P score. Similarly, HDL cholesterol levels showed a significant positive correlation, and triglyceride levels a negative correlation, with AUDIT total score and AUDIT-C score, but not AUDIT-P score. These patterns were previously observed for alcohol consumption (
9). We could speculate that these differences may be linked to socioeconomic status. Alcohol consumption is often higher in individuals with higher socioeconomic status (
45), whereas alcohol-related problems, such as binge drinking (
46) and alcohol-related mortality (
47), are more prevalent in individuals with lower socioeconomic status. Furthermore, individuals with low socioeconomic status are more likely to have alcohol use disorders with psychiatric comorbidities (
48). Consistent with this idea, we find positive genetic correlations between AUDIT-C score and education, a trait correlated with socioeconomic status (
49), and positive genetic correlations between AUDIT-P score and psychopathology. Our findings provide further evidence that different dimensions of alcohol use associate differently with behavior and that these differences may have a biological underpinning.
A clinical diagnosis of an alcohol use disorder is often required to define cases for genetic studies. An alternative strategy would be to use AUDIT to infer alcohol use disorder case status; however, it has not been clear whether and how to perform meta-analyses between AUDIT scores and alcohol dependence. A GWAS meta-analysis for AUDIT score and alcohol dependence could be simplified if a threshold could be used to define cases and controls based on AUDIT score, an approach that was used by Mbarek et al. (
50). We have provided empirical evidence about genetic correlations between AUDIT score and alcohol dependence using dichotomized AUDIT scores and found thresholds for AUDIT score that produced high genetic correlations with alcohol use disorders (see
Figure 3). Genetic correlations increased as the upper threshold for cases was made more stringent, although the standard errors for all of these estimates were overlapping. The genetic correlation with alcohol dependence appeared to become asymptotic when case status was defined as ≥12; therefore, this threshold could be used to define case status. We also considered various thresholds for defining controls and found that a threshold of ≤4 produced a high genetic correlation with alcohol dependence while also retaining the largest number of subjects.
Our study is not without limitations. The AUDIT specifically asks about the past year, and thus it may not capture information on lifetime alcohol use and misuse. This is suboptimal for genetic studies, because it effectively measures a recent state rather than a stable trait. Measures capturing drinking and alcohol use disorders across the lifespan may be preferable. Also, although the mean AUDIT-C score was 4.24, the mean AUDIT-P score was considerably lower (0.75). Thus, we were not able to perform a more refined categorization (e.g., three subsets: consumption [items 1–3], dependence [items 4–6], hazardous use [items 7–10]), as fewer individuals endorsed the items comprising the AUDIT-P (see Table S7 in the online supplement). Furthermore, our study uses data from the UK Biobank and 23andMe research participants, who were volunteers not ascertained for alcohol use disorders, and hence our findings may not generalize to populations that show higher rates of alcohol use and dependence. Additional alcohol-related phenotypes (e.g., age at first use and patterns of alcohol drinking, including binge drinking) could be used in subsequent genetic studies to identify additional sources of genetic vulnerability for alcohol use disorders. Lastly, we offered guidelines to identify cases to use in genetic studies of alcohol use disorders (i.e., an AUDIT score ≥12) based on genetic correlations; however, these recommendations are not intended to determine thresholds for diagnosing dependence in a clinical setting. Future studies will be able to test whether using the AUDIT as a surrogate for alcohol use disorders will be beneficial for gene discovery. In addition, several studies have argued that lower thresholds should be used for females, which has not been addressed in the present study.