Posttraumatic stress disorder (PTSD) is a common and debilitating psychiatric condition with a lifetime prevalence of 7% among adult Americans (
1) and >25% among combat veterans (
2,
3). PTSD may occur following the experience of, or exposure to, a life-threatening event and is characterized by intrusive thoughts or memories, negative alterations in cognition and mood, hyperarousal, and avoidance (
4). Cognitive deficits in executive functions may manifest in PTSD as well (
5–
7).
In healthy individuals, these cognitive capacities are associated with the functioning of large-scale cortical networks (
8,
9), which have received particularly heavy study using resting-state functional MRI (fMRI) connectivity (
10,
11). Within this framework, the brain is typically parcellated into several well-established networks: visual, somatosensory, ventral attention, dorsal attention, frontoparietal control (i.e., executive), and default mode. Studies of PTSD have in turn noted abnormalities within the default mode network and between the default mode and both the ventral attention and frontoparietal control networks (
12,
13). Despite this pioneering work, fMRI remains a tool with limited clinical utility, as it is not directly translatable to a real-world clinic setting.
Electroencephalography (EEG), by contrast, is a less expensive neuroimaging modality providing sub-millisecond temporal resolution. However, EEG voltage, measured at the scalp, reflects the summation of many neuronal sources—predominantly swaths of pyramidal neurons, of varying distances and cortical orientations, propagating current through tissues of inhomogeneous impedances (
14). Thus, neural sources are attenuated and dispersed upon reaching the scalp, confounding whether scalp EEG channels are detecting unique or common sources. These effects of volume conduction limit the spatial resolution of EEG and contribute to making the determination of the neuronal sources’ locations imprecise.
This “signal smearing” problem has stymied the EEG investigation of PTSD using resting-state source-space connectivity analyses for some time. However, considering the advances resting-state fMRI has made in our understanding of network structure, dynamics, and dysfunction across clinical populations (
15–
17), use of new methods that enable EEG-based resting-state connectivity research by mitigating volume conduction could make substantial inroads in achieving clinical deployability of connectomic research.
Recently, a method was reported for alleviating effects of volume conduction, which can reveal distributed resting-state networks that share similarities with those observed with fMRI. First developed in magnetoencephalography (MEG) and then validated in EEG (
18,
19), this method of orthogonalized amplitude correlation is, by design, insensitive to the trivial, zero-phase-lag correlations attributable to volume conduction. The method relies on correlations between oscillatory signals’ instantaneous amplitude across regions (termed power envelopes) and is furthermore distinct from connectivity measures using coherence and phase, which rely on the precise lags between signals in different regions.
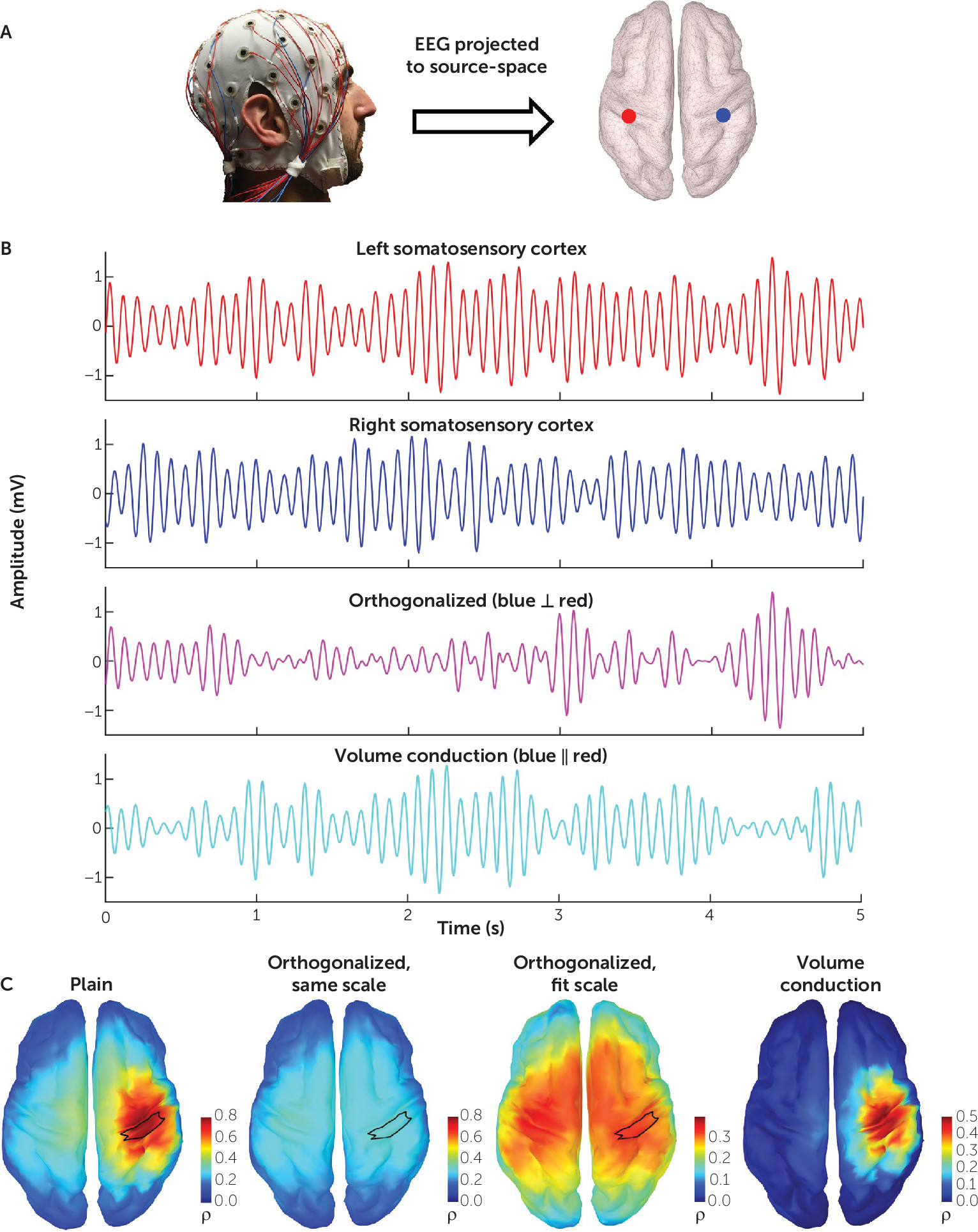
To calculate orthogonalized power envelope correlations, the frequency-band-limited EEG time series is first projected onto the cortical source-space, as shown in
Figure 1A. By way of illustration, we demonstrate the effect of orthogonalization on connectivity by examining two signals in source space from the left and right somatosensory cortex (
Figure 1B). Orthogonalization yields a new signal free of zero-phase-lag effects, representative of the underlying physiological covariation. Volume conduction resulting from this zero-phase lag can likewise be separated by subtracting the orthogonalized signal from the original.
Figure 1C demonstrates the striking effect of orthogonalization on connectivity. Using the right somatosensory cortex as a seed, the whole-brain connectivity map generated from the original signals demonstrates a focal effect at the seed, attributable to volume conduction. Calculating this connectivity using the orthogonalized signal and maintaining the same color scale depicts how volume conduction washes out the physiological signal. Adjusting the threshold reveals the connectivity topography of the somatosensory network. Subtracting the orthogonalized connectivity from the original illustrates the necessity of mitigating volume conduction’s effects.
Importantly, individual differences in MEG power envelope connectivity have been found to correlate with individual differences in fMRI connectivity (
20). This relationship is not isomorphic, however—there is no single MEG or EEG carrier frequency that produces 1:1 mapping to connectivity produced by fMRI. Rather, network topographies generally similar to those of fMRI emerge after applying this power envelope connectivity method to MEG or EEG data, demonstrating that this orthogonalization method can resolve at least some of the same underlying neurophysiological mechanisms behind fMRI networks (
20). Animal work also demonstrates a relationship between power envelope–based connectivity and synchronized blood flow changes across brain regions, which form the basis of fMRI connectivity (
21).
Here, using resting-state EEG data, we first validate the orthogonalized power envelope connectivity method in a sample of healthy civilians and then apply it to a large and demographically homogeneous sample of combat veterans with PTSD (compared with combat-exposed healthy veterans). Using these data, we sought to understand connectomic dysfunction in patients and relate it to specific neurophysiology (e.g., carrier frequency). Once PTSD-related dysconnectivity was identified, we then sought to determine whether it related to clinical or cognitive features of the illness.
Methods
The EEG connectivity method validation study was conducted between March 2015 and October 2016 at Stanford University, and the two-site clinical study was conducted between January 2012 and March 2017 at Stanford University/Veterans Affairs (VA) Palo Alto Health Care System and New York University. Participants provided written informed consent at their respective sites, and the study protocols were approved by each of the institutional review boards.
Participants
In the method validation study, 36 medication-free healthy civilian participants were recruited from the local Stanford community. These participants had never met lifetime criteria for DSM-defined psychiatric disorders, as assessed with the Structured Clinical Interview for DSM-IV (SCID) (
22). They likewise had no history of neurological injury or head trauma and had very low levels of symptoms on self-report clinical scales (
Table 1).
Participants in the clinical study (
Table 2) were 201 combat-exposed U.S. veterans who took part in Operations Enduring Freedom (Afghanistan) or Iraqi Freedom/New Dawn (Iraq). Recruitment was conducted through flyers, social media, and direct contact through VA records. Military service was confirmed using government-issued military or veteran identification and Department of Defense form 214 (discharge document). A diagnosis of full or subthreshold PTSD (three of four clusters met) was made using the Clinician-Administered PTSD Scale (CAPS) for DSM-5 (
23) by a licensed doctoral-level psychologist. We included full and subthreshold PTSD both because a high degree of impairment has been found in subthreshold PTSD (
24,
25) and in order to include a broader range of clinical participants and avoid diagnostic discrepancies related to the changes in diagnostic criteria for PTSD across DSM-IV and DSM-5 (
26–
28). Diagnosis of other comorbid conditions, such as major depressive disorder, was done using the SCID. All diagnoses were confirmed in consensus clinician meetings. A history of traumatic brain injury (TBI) was determined on the basis of whether loss of consciousness occurred after a combat-related head trauma. In addition to the CAPS and the SCID, participants completed the Beck Depression Inventory (BDI) to assess depressive symptoms. All medication dosages for the PTSD sample were stable for 3 months before study enrollment, and the control sample was free of psychiatric medication.
Cognitive Assessments
A comprehensive computer-administered neurocognitive battery was used to assess multiple domains, emphasizing executive functions, including working memory (digit span task), inhibition (go/no-go task), and shifting (Trail Making Test, parts A and B) (
29,
30). Impairments of these domains in PTSD are common (
6), and we chose representative measures in each to examine the behavioral correlates of differences in functional connectivity. For additional details, see the Supplemental Methods section in the
online supplement.
EEG Acquisition, Preprocessing, and Source Localization
EEG recordings were acquired with a BrainAmp DC amplifier (sampling rate, 5 kHz; measurement range, ±16.384 mV; cutoff frequencies of the analog high-pass and low-pass filters, 0 and 1 kHz) and the EasyCap EEG recording cap with 64 sintered Ag-AgCl electrodes (Brain Products GmbH, Germany). The electrode montage followed an equidistant arrangement extending from below the cheekbone back to below the inion. Electrode impedances were kept below 5 kΩ. An electrode attached to the tip of the nose was used as the reference. Participants were seated on a comfortable reclining chair and were instructed to remain awake and let their mind wander in the eyes-closed paradigm and then to fixate on a given point in the eyes-open paradigm, each for 3 minutes. Recordings were immediately assessed for quality using a custom MATLAB (release R2014b; MathWorks, Inc., Natick, Mass.) script and were rerun if necessary. Details of our preprocessing and source localization approach are described in the online supplement. The resulting EEG data were then filtered into four canonical frequency ranges: theta (4–7 Hz), alpha (8–12 Hz), beta (13–30 Hz), and gamma (31–50 Hz).
Calculation of EEG Connectivity
The analytical signal, which is the complex-valued time series containing information about phase and amplitude, of each vertex of source-space was separately and iteratively orthogonalized with respect to all other vertices. Power envelopes were then calculated from each of the orthogonalized analytical time series, and the natural logarithm of these envelopes was taken to render them more normal (
18). A Pearson’s correlation coefficient between the log-transformed power envelopes was obtained for each vertex pair. For comparison, connectivity matrices for the raw power envelopes, calculated from non-orthogonalized analytical time series, were similarly computed. This workflow is illustrated in Figures S1–S3 in the
online supplement. Additionally, a well-annotated MATLAB script used to perform these connectivity analyses is included in the
online supplement.
Connectivity was calculated among 31 regions of interest in Montreal Neurological Institute space (see Figure S4 in the
online supplement), derived from an independent parcellation of resting-state fMRI connectivity using independent components analysis from 38 healthy subjects applied in a previous study (
31). This was done to ground our EEG regions of interest in a commonly used, fMRI-derived definition of the major cortical connectivity networks while maintaining region-of-interest sizes large enough to be transformed for use in the lower-resolution electrophysiological context (
10,
32).
For each region-of-interest pair, the Fisher z transformations of the Pearson correlations of each pair of vertices within each of the regions of interest were averaged to determine the connectivity of that region-of-interest pair. As a result, 465 unique region-of-interest pairs (excluding self-connectivity) were computed in each of the four frequency bands, in each of the two resting paradigms (eyes open and closed), and in each of the two computational methods (plain and orthogonalized).
Statistical Analysis
Mean region-of-interest pair connectivity values were analyzed in parallel linear mixed-effects (LME) models with PTSD diagnosis as a categorical predictor, collection site as a covariate, and random intercept for participant. The p values for this analysis were corrected using a single false discovery rate (FDR) adjustment (pFDR, 1860 comparisons<0.05; Benjamini-Hochberg method) that included all regions of interest and frequency bands. Outliers in connectivity measures exceeding the median ±3 times the interquartile range were excluded (mean=2.7 subjects, SD=2.6).
To determine whether subjective clinical symptoms or behavioral measures of cognitive domains mapped onto measures of connectivity, we used the surviving region-of-interest connections from the above LME model as response in another LME model including site as covariate and random intercept for participant. The cognitive domain measures, CAPS total score, CAPS subscale scores, and BDI total score were set as separate predictors. Nonresponses or timed-out responses in the behavioral data were excluded (four, three, and two subjects in the working memory, inhibition, and shifting tasks, respectively).
Results
Method Validation Study in Healthy Control Subjects
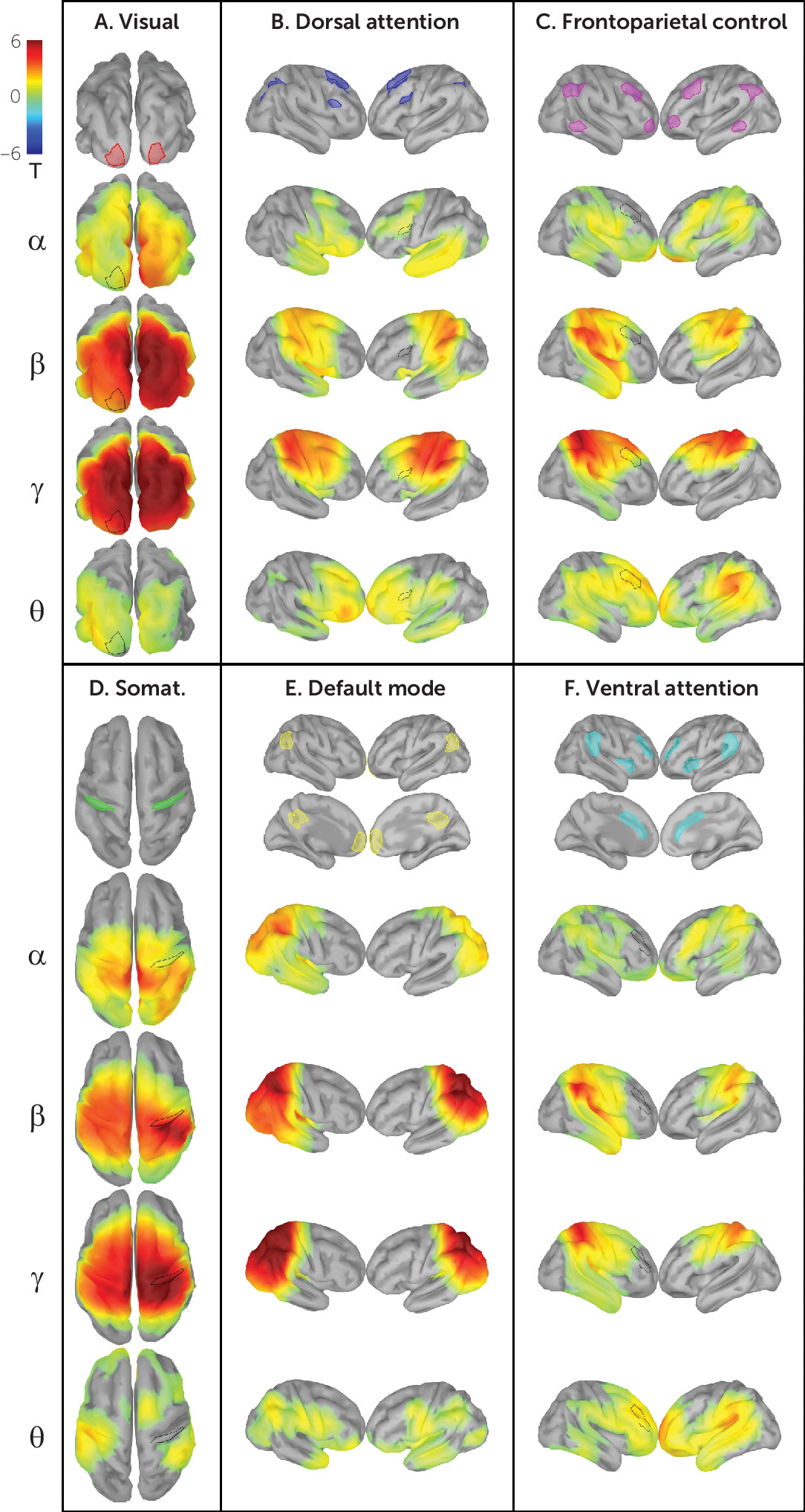
Figure 2 shows network connectivity patterns attained in our validation sample using power envelope connectivity. As predicted from previous work developing and validating this method (
18,
19), the expected connectivity patterns emerged only after orthogonalizing power envelopes to suppress zero-phase-lag volume conduction. By seeding various constituent regions of interest of the major cortical networks and calculating the t test for correlation ≠ mean brain-wide correlation (p
FDR, 3000 vertices<0.05, two-tailed), connectivity patterns generally consistent with those observed in fMRI studies emerged—for example, the visual, dorsal attention, frontoparietal control, somatosensory, default mode, and ventral attention networks (
Figure 2). Greater intranetwork connectivity as compared with extranetwork connectivity (
Table 3) is seen in all networks in various frequency bands (t test for mean of region of interest to within-network vertices ≠ mean of region of interest to extranetwork vertices, p
FDR, 24 network-frequency band pairs<0.05, two-tailed). As expected, early sensory networks (visual and somatosensory) are highly intraconnected across nearly all frequency bands, and the default mode network demonstrates canonical intraconnectivity in the alpha and beta bands. Figure S5 in the
online supplement illustrates more canonical distributed topographies achieved by calculating the t statistic of mean region-of-interest connectivity >0 rather than a brain-wide mean. Similarly, Table S1 in the
online supplement describes the intranetwork minus extranetwork connectivity obtained by this method, which produces the same significant network-frequency band pairs as those listed in
Table 3. Diffuse extranetwork connectivity is observed in most network-frequency band pairs, likely attributable in part to network overlap unresolved by EEG’s lower spatial resolution (e.g., the apparent overlap of the inferior parietal lobule and angular gyrus in the default mode and frontoparietal control networks).
Connectivity Differences in PTSD
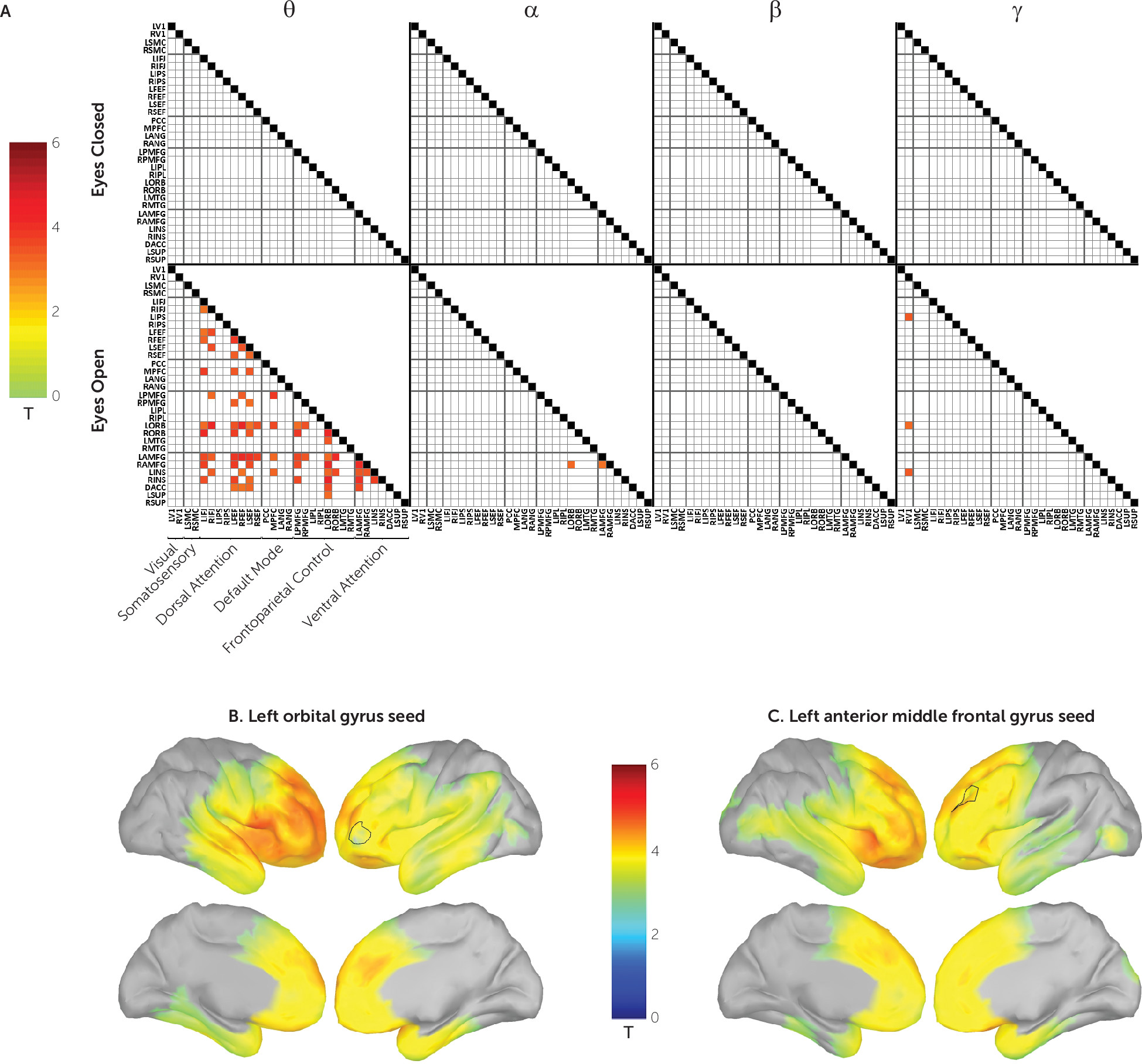
Next, we tested, at each of the four carrier frequencies, whether power envelope connectivity was perturbed in participants with PTSD. Seventy-four region-of-interest pairs survived correction for multiple comparisons (
Figure 3A). These were found exclusively in the eyes-open condition and predominantly in the theta carrier frequency. Brain region pairs demonstrated positive connectivity in the control group and underconnectivity in the PTSD group; this contrast is illustrated among selected regions of interest in Figure S6 in the
online supplement. In all cases, these represented hypoconnectivity in the PTSD group. The majority of significant region-of-interest pairs were connected to the orbital and anterior middle frontal gyri. The whole-brain FDR-corrected vertex-wise PTSD hypoconnectivity maps for these two regions are shown in
Figure 3B and
3C. As can be seen, hypoconnectivity is particularly pronounced across the lateral and medial frontal cortex as well as the inferior parietal lobe.
In contrast to these results, similar group comparisons of regional power spectral density in each of the frequency bands, using the same false discovery rate correction, failed to yield any significant findings (data not shown). Hence, our results are specific to power envelope connectivity, almost exclusively within the theta frequency band, and are not evident if one examines only the mean spectral power of that frequency band (theta included). Moreover, consistent with expectations about the importance of orthogonalization during the calculation of power envelope connectivity, an analysis similar to that described above conducted on non-orthogonalized power envelopes failed to yield any significant group difference results (data not shown). Global mean connectivity was also examined in both clinical groups, and no significant differences were found in any band or paradigm.
To assess the detection power of the power envelope connectivity as a potential biomarker, we used the orthogonalized connectivity values as features to train a machine-learning classifier for distinguishing PTSD patients from trauma-exposed control subjects. Classification performance was evaluated using a leave-one-out cross-validation procedure. The receiver operating characteristic curve (ROC) (see Figure S7 in the online supplement) shows promising classification performance (area under the curve [AUC]=0.898, sensitivity=80.2%, specificity=84.9%). To further confirm the biomarker’s generalization capability, we also performed a 10×10-fold cross-validation procedure. The ROC analysis shows that our discovered biomarker still provided good classification performance (AUC=0.813, sensitivity=76.3%, specificity=74.9%; see the Supplemental Methods section in the online supplement), demonstrating its generalization capability for identifying PTSD patients.
Clinical and Behavioral Significance of Power Envelope Dysconnectivity
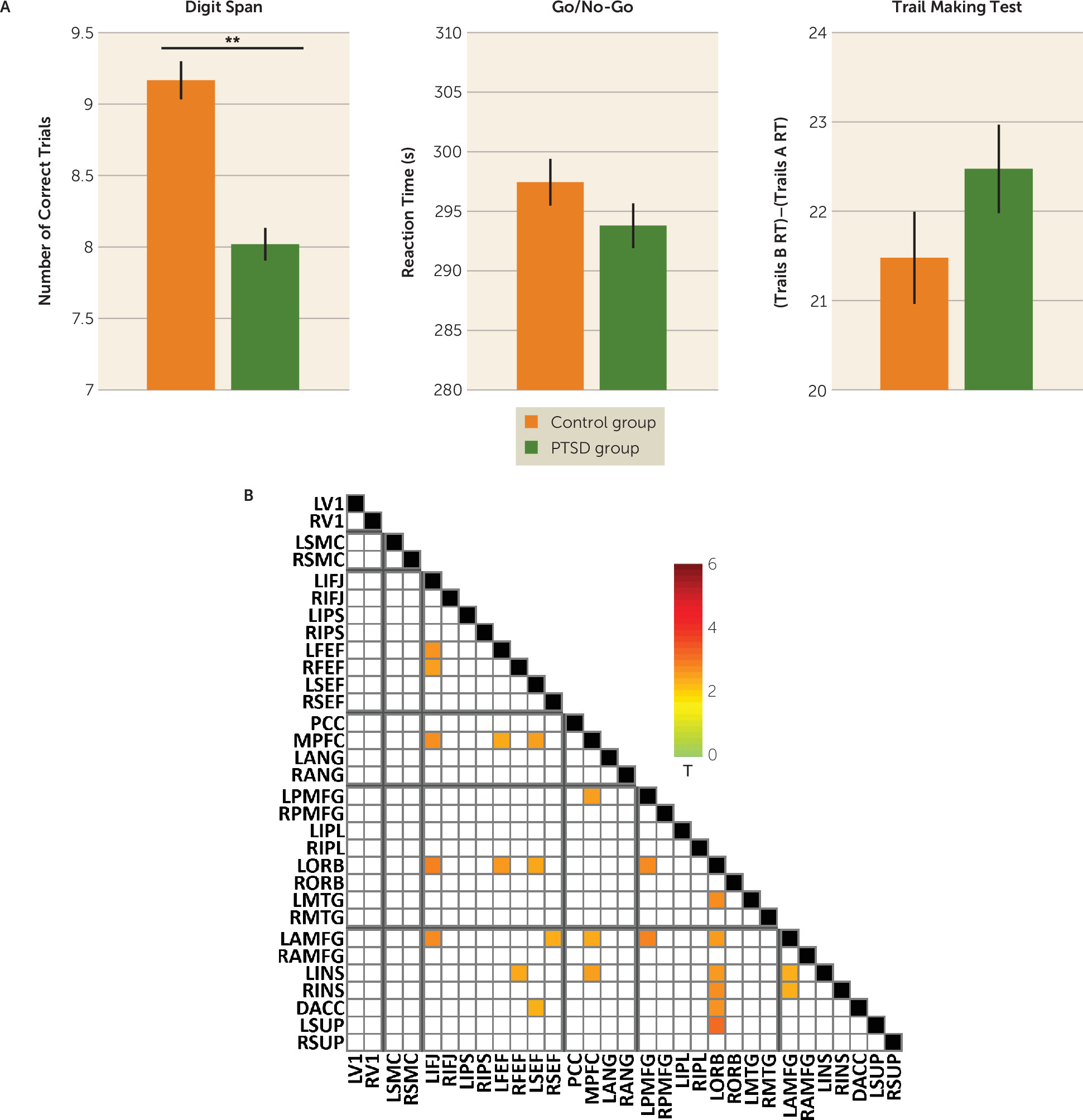
To understand the relevance of the heavily prefrontal and chiefly theta-based connectivity abnormalities in cognition in PTSD, we correlated performance on tasks of executive functions (working memory, inhibition, and shifting) with connectivity in the significant region-of-interest pairs that emerged from the analysis described above. The PTSD group performed significantly worse than the control group on the digit span task (
Figure 4A). Across both groups, individual differences in digit span performance furthermore correlated with individual differences in power envelope connectivity for 25 region-of-interest pairs of the 74 that showed a significant group difference, including within-network pairs in the dorsal attention, frontoparietal control, and ventral attention networks (
Figure 4B). No significant group differences were found for other cognitive measures.
We additionally tested whether the observed connectivity abnormalities were related to clinical features of PTSD. However, measures of symptom severity on the CAPS total and subscale scores, as well as on the BDI, did not significantly correlate with connectivity among the region-of-interest pairs identified in the group contrast above. Occurrence of comorbid major depression or TBI also did not account for significant differences in connectivity among the region-of-interest pairs identified above. Similarly, while 26.4% of the PTSD group used daily or as-needed psychotropic medications (see
Table 2), use of psychotropic medication did not account for significant differences in connectivity.
Discussion
We have validated the use of orthogonalized power envelope connectivity with resting-state EEG data as a powerful tool that can reveal general network connectivity patterns otherwise obscured by volume conduction. Doing so in a large and demographically homogeneous group of combat-exposed veterans revealed marked prefrontal hypoconnectivity in orthogonalized power envelope connectivity predominantly using a theta carrier frequency and exclusively in the eyes-open condition. Moreover, this connectivity abnormality was related to an objective cognitive impairment but not subjective clinical symptoms. Critically, group differences were not found when using either non-orthogonalized power envelope connectivity values or simple regional spectral power density. The latter is a commonly used measure in prior resting-state EEG studies and thus illustrates the contrast between our approach and those used previously (
33).
Connectivity abnormalities in PTSD have been established in several resting-state fMRI studies (
34). A study of male combat veterans found that severity of PTSD symptoms correlated with hypoconnectivity between the medial prefrontal cortex and a region of interest within Brodmann area 10 (coinciding with our orbital gyrus region of interest), and worse overall neuropsychological performance mapped onto reduced functional connectivity in constituent regions of interest of the ventral attention and default mode networks as well (
15). A civilian study similarly found hypoconnectivity within the ventral attention and default mode networks in PTSD (
13). Thus, several of the connectivity abnormalities we detected have previously been implicated in PTSD using fMRI.
Although several investigations of resting-state fMRI connectivity have been performed in PTSD, there is a dearth of source-space resting-state EEG connectivity studies in PTSD, especially in veterans. One recent resting-EEG-derived functional connectivity study (
35) found that nearly all global network indices of connection strength, clustering coefficient, and efficiency were reduced in PTSD, and in all frequency bands. Indeed, virtually all nodal pairs across the whole brain were underconnected in the PTSD group. However, that study differed from ours in several ways, including in their examining only civilian PTSD and using phase-locking value as a connectivity measure, whereas we examined amplitude-based connectivity. Moreover, phase-locking value connectivity can be highly susceptible to violations of the assumption of signal stationarity, whereas time-point-by-time-point power envelope orthogonalization is not (
36). It is distinctly possible that volume conduction, insufficiently mitigated by the phase-locking value approach, may also be the source of the observed global connectivity abnormalities across all bands.
Predominance of connectivity differences for theta carrier frequency power envelope connectivity may be related to frontal midline theta signals that are thought to mediate functional network interactions (
37). In rodents, frontal midline theta reflects hippocampal influence on the neocortex (
38). In humans, it is associated with different aspects of working memory (
39), including maintenance (
40). These findings are in line with our current mapping between working memory maintenance (i.e., digit span task) and prefrontal resting connectivity. Furthermore, it is suggested that theta oscillations, especially frontal midline theta, play an integral role in network interaction (
41) and cognition (
42). In a simultaneous fMRI-EEG memory task (
43), an inverse relationship between theta power and default mode network activation was observed, and in a study of generalized anxiety disorder (
44), frontal midline theta coherence differed significantly between the patient and control groups. Whereas the exact mechanisms of interaction and their variation in clinical populations remains an open question, our results support these broader associations between theta carrier frequency and cognition and demonstrates their relevance for PTSD.
The theta and eyes-open predominance of our connectivity abnormalities are also noteworthy considering that EEG spectral power is greatest in the alpha band and when the eyes are closed (
45), as we have likewise observed in these data. Furthermore, power envelope connectivity is greatest in the alpha to low beta range (
18,
19). Thus, the deficit in the PTSD group does not reflect an abnormality simply where the signal is strongest, but rather our findings reinforce the neurophysiological and clinical significance of theta-range connectivity.
Regarding the specificity to the eyes-open condition, both EEG and fMRI studies, including studies conducted in complete darkness, demonstrate that intrinsic resting networks of the brain organize differently between eyes-open and eyes-closed paradigms (
46,
47). In eyes-open EEG, a selective increase in power and coherence is observed in the frontal and temporal cortices. In fMRI, the eyes-open minus eyes-closed contrast is maximal in the frontal and temporal cortices as well, with notable cluster maxima in the orbital and middle frontal gyri (
48). An MEG study examining functional connectivity differences between the paradigms found higher global efficiency in theta and alpha bands in the eyes-open state and higher centrality in nodes of higher betweenness in theta eyes open (
49). These phenomena are thought to reflect the brain’s functional reorganization from an internally focused state (eyes closed) to one focused on the external environment (eyes open), which affects interaction of the default mode, frontoparietal control, and ventral attention networks.
Further exploration of theta band connectivity in PTSD serves as a promising avenue for future research. Given the translatability of EEG to the clinical setting and the emergence of a robust connectomic profile of brain regions amenable to transcranial magnetic stimulation, answers to questions of whether these profiles change with treatment or predict treatment outcome are within reach.
Our study has some limitations. It investigated solely the neural correlates of PTSD among predominantly male combat veterans. Although this is beneficial in maintaining demographic homogeneity for this analysis, it may limit our findings’ generalizability regarding PTSD originating from other traumas (PTSD in civilians occurs predominantly in females). PTSD is not a unitary construct (nor is any psychiatric disorder). However, our goal here is to introduce and demonstrate one aspect of the clinical utility of an important new EEG connectivity method, focusing on features that are seen across the broader PTSD population. The severity of PTSD symptoms was also moderate in this sample. The majority of participants with PTSD were still serving or were gainfully employed; findings may be different in a highly symptomatic or psychiatrically disabled sample. However, this method’s ability to detect connectivity differences in high-functioning individuals with PTSD alludes to its potential when applied to typical PTSD populations.
The use of individual head models derived from participants’ MRIs may generate more accurate spatial filters. Use of template brain head models, however, will make our results more generalizable to clinic-based EEG acquisition, in which MRI imaging is often not feasible or cost-effective.
Conclusions
We established the relevance of prefrontal theta band power envelope connectivity for PTSD, as well as cognitive dysfunction in the disorder. By employing a novel analytic method (power envelope orthogonalization) that mitigates volume conduction, it was possible to use resting-state EEG connectivity analyses to reveal a robust connectomic profile of PTSD. Moreover, identification of these connectivity abnormalities sets the stage for nearer-term clinical translation of brain connectivity research, given the greater ease of use and lower cost of EEG compared with conventional fMRI methods.
Acknowledgments
The authors thank Dr. Joerg Hipp for his advisement on implementing the orthogonalization method.