Psychostimulant medication, such as methylphenidate, is a first-line pharmacological treatment for children with attention deficit hyperactivity disorder (ADHD) (
1), and its short-term efficacy is well established (
2–
5). However, the long-term benefits of methylphenidate use remain unclear; current treatment guidelines for ADHD state that there is only evidence for effectiveness of up to 2 years of treatment with methylphenidate and recommend that the need for continued treatment be reviewed annually (
1,
6–
8). Nevertheless, 60% of children who start stimulant treatment continue to use it beyond 2 years (
9,
10). Indeed, stimulant use over several years has been shown to be increasingly common, across adolescence and into adulthood (
11), perhaps as a consequence of the ease of use of long-acting methylphenidate preparations (
12) and of the increased awareness that ADHD often persists into adulthood (
13). The long-term use of methylphenidate, however, contrasts heavily with the lack of data on long-term benefits of stimulant use.
The longest placebo-controlled randomized trial to date was 28 weeks, and it showed a positive effect of methylphenidate on reduction of hyperactivity symptoms (based on parent and teacher ratings of an earlier conceptualization of ADHD that emphasized hyperactivity [
14]). From the Multimodal Treatment of ADHD (MTA) study (
15), a randomized controlled trial comparing four different treatment strategies, we know that methylphenidate, optimally titrated over the course of 14 months, is superior to other treatments: it was found that children taking stimulants alone or combined with behavioral treatment did better than children who received routine community care (which often included treatment with psychostimulants, albeit not necessarily optimally dosed) or behavioral treatment alone. After the 14-month randomized phase, the MTA study conducted naturalistic and observational follow-ups (
16,
17). The most recent long-term report tracked 476 children for 12 to 18 years after they started the controlled phase of the study; the authors concluded that those still taking stimulant medication in clinical practice fared no better in the reduction of symptoms or in social functioning than those who had stopped taking medication (
18). This finding was in line with earlier follow-up reports of the MTA study (
16,
17), and it raises questions about whether long-term medication treatment continues to be beneficial or needed. In a randomized study (
19,
20) comparing three treatment modalities (methylphenidate alone, methylphenidate plus multimodal psychosocial treatment, and methylphenidate plus attention control psychosocial treatment), the benefits of methylphenidate remained stable over a 2-year period. However, the study design did not allow for ruling out effects of the natural course of ADHD on long-term outcomes because of the lack of a control group. That is, children who were treated with methylphenidate were not compared with children who received placebo or children who received no treatment. Thus, current evidence for the long-term benefits of methylphenidate is limited to a treatment duration of 2 years at best (
19–
22), although some studies indicated positive results on substance use risk for patients receiving stimulant medication for more than 2 years (
23,
24).
Given the high number of children and adolescents using methylphenidate for many years, and the recommendation in several guidelines for annual reconsideration of continuing medication, there is a pressing need to investigate to what extent continued use is still of benefit. Obviously, conducting long-term placebo-controlled trials is not viable to address the long-term effectiveness of methylphenidate, as this would require withholding treatments for a long period. That is why we chose an alternative design, a double-blind randomized placebo-controlled discontinuation design. Children and adolescents who were treated in regular clinical practice and had been using methylphenidate for at least 2 years were randomly assigned to either continuation of methylphenidate or gradual withdrawal to placebo. To our knowledge, there have been no studies using a discontinuation design to investigate the effects of stopping methylphenidate in children and adolescents.
The primary objective of this study was to investigate whether methylphenidate remains beneficial after 2 years of use. To enhance the external validity, we included participants who were on dosages as typically prescribed in clinical practice rather than necessarily the most optimal ones. We aimed to test the hypothesis that continued use of methylphenidate is superior to placebo with regard to ADHD symptom severity in two settings, at home and at school. As a secondary objective, we investigated the proportion of individual children who would or would not benefit from continued long-term use of methylphenidate. A final objective was to assess the safety of withdrawing methylphenidate.
Methods
This was a randomized double-blind placebo-controlled two-center discontinuation trial that compared continued use of methylphenidate for 7 weeks with gradual withdrawal to placebo. If a participant’s clinical condition necessitated breaking the blind, study participation ended.
Participants
The study recruited children between 8 and 18 years of age who had been using methylphenidate as prescribed in clinical practice in any dosage or form for 2 years or longer. If a child had stopped the medication during, for instance, a weekend or a school holiday, they could still participate if the period of not using methylphenidate had not exceeded 2 continuous months during the past 2 years. During the past 4 weeks, participants should have used extended-release methylphenidate at either 36 mg or 54 mg per day. Children who were originally not using 36 or 54 mg/day of extended-release methylphenidate could switch to one of these dosages, whichever was the closest to the dosage they were already using, for at least 4 weeks to allow them to participate in the study.
Exclusion criteria included the intent to start new psychosocial or pharmacological therapies during the study period, an IQ below 70, inability on the part of the child or the parents to understand or comply with the study protocol, and presence of a severe medical or psychiatric condition the treatment of which would have interfered with the study. Participants could not start during or 7 weeks before the summer vacation period, to allow for investigating discontinuation effects during regular school attendance.
Participants were recruited from two outpatient child and adolescent psychiatry centers: Accare in the northern Netherlands and Karakter University Center in Nijmegen. We included children with an IQ over 70 based on a known IQ test or regular education attendance. Meeting formal criteria for ADHD was not an inclusion criterion given that children did not receive a standardized research diagnostic assessment procedure prior to methylphenidate initiation. Parents and children age 12 and older both provided written informed consent. For children under age 12, the parents provided written informed consent and the children provided oral assent, in accordance with Dutch medical ethics laws. On the consent forms, parents could separately give permission to obtain teacher ratings, which was optional. The study was approved by national and local institutional review board committees.
Interventions
After consent had been obtained, participants were randomly assigned in a 1:1 ratio to either continued active medication at the same maintenance dosage for 7 weeks or to gradual withdrawal to placebo over a 3-week period followed by 4 weeks of complete placebo. The dosage reduction schedule was the same for both those who had been using 54 mg/day and those who had been using 36 mg/day of extended-release methylphenidate at the beginning of the study: 36 mg/day during week 1, 27 mg/day during week 2, 18 mg/day during week 3, and placebo for weeks 4 through 7. Participants used the same schedule for taking their medication as they did before the trial (e.g., daily or with weekend stops). Participants could continue using any comedication or receiving any kind of psychosocial interventions that they were already using before the trial.
The study medication consisted of an overencapsulation of methylphenidate in an osmotic-controlled release oral delivery system (18 mg, 27 mg, 36 mg, and 54 mg). The overencapsulation was backfilled with lactose monohydrate for blinding purposes. Matching placebo capsules contained only the filler.
Outcomes
The primary outcome measure was the total score on the investigator-administered ADHD DSM-5 rating scale (ADHD-RS). This is an adapted version of the ADHD Rating Scale–IV (
25), in accordance with the changes in ADHD criteria introduced in DSM-5 (
26). This instrument contains 18 items on inattentive and hyperactive-impulsive symptoms (scored on a 4-point scale, where 0=never, 1=sometimes, 2=often, 3=very often) and assesses ADHD symptom severity over the past week. We used the total score, which is the sum score of all 18 items. The ADHD-RS inattentive and hyperactivity-impulsivity subscales were used as secondary outcome measures, each consisting of the sum score of the respective nine items. We obtained ADHD-RS ratings at baseline and after 7 weeks, or at time of dropout for participants who withdrew from the study.
Other secondary outcome measures included the investigator-rated Clinical Global Impressions improvement scale (CGI-I) (
27) and the Conners’ Teacher Rating Scale–Revised: Short Form (CTRS-R:S) (
28), assessed at baseline (CTRS-R:S only) and after 7 weeks or at the time of study dropout. The CGI-I rated the global improvement or worsening in ADHD symptoms of the participant compared with baseline on a 7-point scale (1=very much improved, 2=much improved, 3=minimally improved, 4=no change, 5=minimally worse, 6=much worse, 7=very much worse), taking into account both the home and school situation according to parent reports. The CTRS-R:S is a teacher-rated 28-item 4-point Likert rating scale that contains items on ADHD and comorbid conditions (i.e., problems with conduct, emotion, anger control, and anxiety) in the school setting. It consists of four subscales: oppositional, cognitive problems/inattention, hyperactivity, and an ADHD index. Here we report only the results on cognitive problems/inattention, hyperactivity, and the ADHD index. Finally, we noted all adverse events that were spontaneously reported to the investigator by the child or parents.
Sample Size
We planned a sample of 120, with 60 in each group. With this sample size, it is possible to detect an effect size (Cohen’s d) of 0.251 with a power of 0.80 and alpha of 0.05, as calculated with the program G*Power, version 3.1. We aimed to include equal numbers of participants on 36 mg/day and 54 mg/day of methylphenidate, as those were the most commonly prescribed dosages of extended-released methylphenidate in the participating outpatient clinics.
Randomization
The trial pharmacy dispensed study medication for either continued active medication or discontinuation according to two separate computer-generated randomization lists, for each dosage. A block-randomization of 6 was used to ensure even groups.
Statistical Analysis
We used the group-by-time interaction of the mixed model for repeated measures (MMRM) to analyze differences in outcome measures (ADHD-RS-5, CTRS-R:S) from baseline to 7 weeks between both randomized groups, using group (continuation or discontinuation) and time point (baseline or 7 weeks) as fixed effects. An unstructured covariance matrix was used. Analyses were conducted on the full data set, which included all participants who received at least one dose of the study drug. In those who had withdrawn from the study, we used ratings that were obtained at the time of study termination. To determine whether age was associated with the effect of discontinuation regarding the ADHD-RS total score, we added a group-by-time-by-age interaction term to the MMRM analysis. We subsequently determined the direction of a significant age effect with a median age split. A chi-square test was used to analyze whether there was a difference between the two groups in the number of participants prematurely withdrawing from the trial. Differences in baseline characteristics between the two randomized groups regarding age, sex, duration of use, start age, and percentage of users of the 36 mg/day and 54 mg/day dosages were analyzed using the chi-square test or Student’s t test as appropriate.
To test whether the proportion of children who worsened differed between the two randomized groups, we used a chi-square test on dichotomized CGI-I scores, comparing “much worse” and “very much worse” (coded as worsened) with all else (coded as not worsened). We also calculated the number needed to treat to avoid such a worsened outcome. Adverse events were analyzed using Fisher’s exact test for a comparison between groups. The significance threshold for all analyses was a p value of 0.05. As a measure of effect size, we used Cohen’s d (
29). An effect size around 0.20 is considered small, around 0.50 medium, and around 0.80 large.
Results
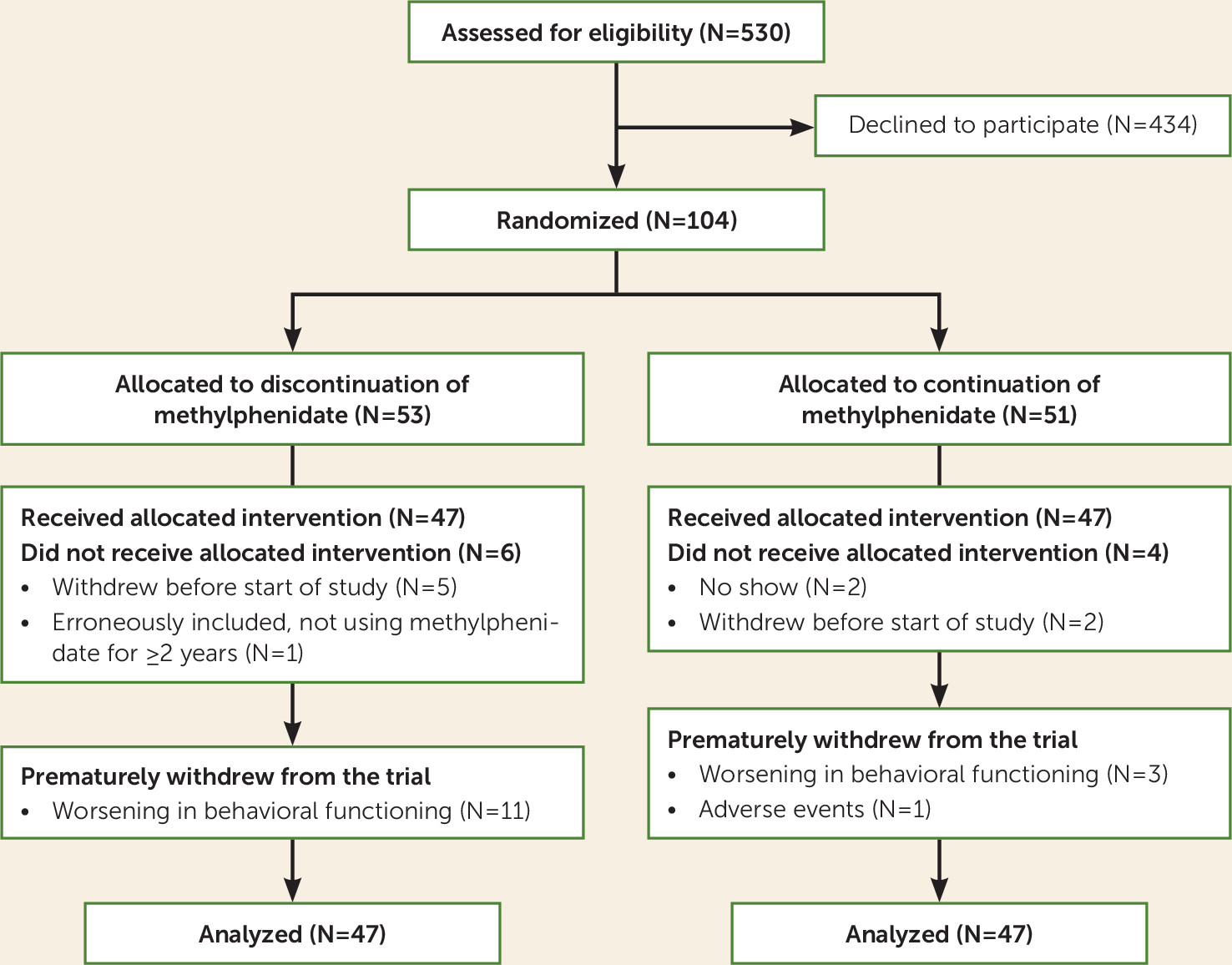
The CONSORT diagram (
Figure 1) summarizes the flow of participants through the study. Given recruitment difficulties, we were able to recruit only 94 of the intended 120 participants in the fixed time frame. With this lower sample size, it is possible to detect an effect size (Cohen’s d) of 0.284, still representing a small effect, with a power of 0.80 and alpha of 0.05, as calculated with G*Power, version 3.1. Parents of 78 participants also gave permission for us to obtain the CTRS-R:S teacher ratings. A total of 47 participants of the 94 (50%) had switched from another form of methylphenidate to extended-release methylphenidate at 36 or 54 mg/day for 4 weeks to be able to participate in the study. Participants were enrolled from September 2015 to December 2016. Accare recruited 69 participants and Karakter 25. In the discontinuation group, 11 participants (23.4%) terminated the discontinuation prematurely, compared with four (8.5%) in the continuation group, a significant difference (χ
2=3.9, df=1, p=0.049), their time in the trial ranging between 4 and 6 weeks.
Baseline Demographic and Clinical Characteristics
The participants’ baseline characteristics, summarized in
Table 1, were similar for the methylphenidate discontinuation and continuation groups. The two groups did not differ significantly in age, sex, ethnicity, duration of methylphenidate use, start age, or percentage of 36 mg/day and 54 mg/day users at the start of the trial.
Summary of Results
As shown in
Table 2, there was a significant difference between the discontinuation and continuation groups in the level of mean change, from baseline to 7 weeks, in ADHD-RS total score and ADHD-RS inattention subscale score, but not in ADHD-RS hyperactivity-impulsivity subscale score. These group-level analyses indicated that, on average, the ADHD scores deteriorated to a significantly larger extent in the discontinuation group than in the continuation group. However, effect sizes were small. We found that age interacted with the effectiveness (based on ADHD-RS total score) of continuing methylphenidate treatment compared with placebo (F=2.61, df=92, p=0.04). More specifically, the effect of continuing methylphenidate was superior in the group of younger participants (i.e., those below the median age of 13.8 years; F=7.05, df=45, p=0.01, d=−0.45), but absent in the group of older participants (F=0.32, df=45, p=0.57).
Analyses on an individual level showed that CGI-I scores indicated worsening in overall functioning in 19 of the 47 participants (40.4%) in the discontinuation group, compared with seven of the 47 participants (15.9%) in the continuation group, a significant between-group difference (χ
2=6.7, df=1, p=0.01) (see
Table 2). The number needed to treat to avoid worsening was 4.
The MMRM analyses for the CTRS-R:S teacher ratings (
Table 3) showed significant differences with regard to the ADHD index (p<0.001) and the hyperactivity subscale score (p=0.001): the mean change from baseline was significantly larger among participants assigned to the discontinuation group than among those receiving methylphenidate, with medium effect sizes.
Adverse Events
None of the participants had a serious adverse event. One participant in the continuation group withdrew from the study because of adverse events (i.e., headaches, purple spots [on the arms], dizziness, shortness of breath, thirsty, itching, muscle cramps, and weight change). In the discontinuation group, 13.5% reported at least one adverse event, compared with 10.6% in the continuation group (χ
2=0.54, df=1, p=0.46).
Table 4 lists all reported adverse events.
Discussion
With the aim of investigating the benefits of treatment with methylphenidate beyond 2 years, we randomly assigned children and adolescents to either continuation of methylphenidate treatment or gradual withdrawal to placebo, using a double-blind placebo-controlled discontinuation design. Our findings indicate that continued use of methylphenidate is still superior to treatment discontinuation after at least 2 years of use, as assessed by both investigator- and teacher-rated ADHD symptom ratings. We did find a moderating effect of age, indicating that the superiority of continued treatment with methylphenidate is especially present in younger children.
The effect size of investigator ratings (d=−0.23) that we found when methylphenidate as used in clinical practice was discontinued was about one-third of the effect size (d=−0.71) found in a meta-analysis of studies examining the short-term efficacy of methylphenidate (
30). On the one hand, this might suggest that methylphenidate becomes less effective when used over a longer period than in the first weeks of treatment. This would be in line with the long-term MTA data, which also suggest that the effects of methylphenidate become less pronounced in the long run (
16–
18). On the other hand, the lower effect size compared with those from acute studies may be explained by the fact that we studied the effects of discontinuation of dosages of methylphenidate as prescribed in clinical practice, which are typically lower than optimally titrated dosages.
Two additional factors may have led to an underestimation of the effects of methylphenidate withdrawal. First, we applied gradual down-titration instead of directly discontinuing the active medication (although this will have had the benefit of contributing to successful blinding). Second, and more importantly, we acknowledge the large numbers of eligible patients who declined to participate in our trial. Given the acute mode of action of methylphenidate, many patients we approached did not want to participate in our discontinuation trial, as they argued that they “knew it still worked,” based on experiences of stopping briefly or when they forgot to take their medication for a day. As a consequence of this, our sample may have overrepresented participants in whom the effects of methylphenidate were less pronounced, perhaps because of more mild ADHD symptoms or ADHD that was resolving.
The effect size of teacher ratings (d=−0.52) was substantially larger than that of the investigator ratings; it was more in line with the effect sizes observed in trials for teacher ratings when medication is initiated and was mostly driven by effects on hyperactivity. Teachers were recently found to report a lower placebo response than parents did in a placebo-controlled crossover trial with methylphenidate (
31). This may be an explanation for the higher teacher-based effect size compared with that of investigators, as the latter was based on information from parents.
As a secondary objective, we investigated the proportion of individual children who would or would not benefit from continued long-term use of methylphenidate. The CGI-I-based results confirmed the ongoing effectiveness of continued treatment with methylphenidate; the percentage of those who worsened in the placebo group was clearly higher than in the group that remained on active medication (40% compared with 15.9%), which indicates that the number needed to treat is only 4 to avoid worsening. Another indication for the ongoing effectiveness was that, compared to the continuation group, nearly three times as many participants from the discontinuation group dropped out of the study because of worsening of symptoms.
However, the CGI-I results also showed that 40% of the participants who discontinued experienced worsening, whereas 60% did not. Thus, our CGI-I findings indicate that some individual patients may be withdrawn from methylphenidate without deterioration. This supports recommendations of guidelines to periodically assess whether continued methylphenidate use is still needed in a patient or if symptoms have remitted, especially in adolescents, as ADHD symptoms may decrease over time, at least in a subset of patients (
32,
33). The clinical decision to discontinue or continue treatment with methylphenidate should be based on a careful long-term assessment of risks and benefits that goes beyond ADHD ratings and may include functional impairments and environmental risks (
34–
37). One may also argue that regular attempts to optimize the dosage of methylphenidate are warranted.
Our study should be regarded in light of its strengths and limitations. A great strength was the embedding of the study in regular clinical care, having high ecological validity and important clinical implications. As a first limitation, we already discussed the potential impact of the large numbers of eligible patients who declined to participate in our trial. A second limitation may have been the restriction of the study to children using extended-release methylphenidate at 36 mg/day or 54 mg/day at the time of enrollment, not fully covering the diversity in types and dosages of stimulant medication use in ADHD. Unfortunately, logistic hurdles did not allow for including lower or higher dosages. However, we are confident that by allowing participants on different formulations or dosages to switch to extended-release methylphenidate at 36 or 54 mg/day in order to participate in the study, we obtained a group that is most representative of clinical practice. Third, we lack evaluations of participants prior to the discontinuation trial. This would be useful in future studies to compare initial effects of medication to the effects of discontinuation and to identify baseline predictors of long-term effectiveness. Fourth, one might criticize the fact that we did not require participants to have a diagnosis of ADHD based on standardized assessments. Again, however, we argued that the group that we included should reflect clinical practice, in which children are typically being treated with methylphenidate without the use of standardized assessments for ADHD. Fifth, the apparent absence of ongoing effectiveness in older youths may partially be explained by the lessened sensitivity of investigator or teacher ratings of ADHD symptoms, as older youths are less likely than younger children to display overt hyperactivity (
38,
39). Future studies should include self-ratings for adolescents. Finally, we should acknowledge the modest sample size; we cannot exclude the possibility that the differential impact of discontinuation on inattention and hyperactivity-impulsivity symptoms according to investigator and teacher ratings were due to limited statistical power.
Conclusions
Our study suggests that methylphenidate is still an effective treatment after 2 years of use, even if the treatment effect size appeared rather small. One should keep in mind, however, that our sample likely included an overrepresentation of families who suspected that the medication may not have been working well and therefore wanted to try going off medication. Those who declined to participate may have felt more confident that the medication was helpful and may therefore have been unwilling to risk being assigned to the placebo condition. Another factor contributing to the small effect size may have been the fact that the participants were not necessarily on an optimal dosage of methylphenidate before entering the study. Current guidelines include detailed recommendations on how to prescribe methylphenidate, which contrasts heavily with the almost complete lack of recommendations regarding the long-term use of methylphenidate (
1,
6–
8). Nevertheless, the fact that most participants in our study did not experience significant worsening after discontinuation of methylphenidate supports guideline recommendations to periodically assess whether there is a continued need for methylphenidate treatment, for example, by considering a temporary discontinuation of medication in clinical practice to prevent unnecessary long-term medication use. Studies with larger samples should investigate whether there is a subgroup of individuals with persistent ADHD who would clearly benefit from long-term treatment with methylphenidate. Another important suggestion for future studies is to conduct a longer-term discontinuation trial with optimally dosed methylphenidate to investigate its long-term efficacy.