Associations of Benzodiazepines, Z-Drugs, and Other Anxiolytics With Subsequent Dementia in Patients With Affective Disorders: A Nationwide Cohort and Nested Case-Control Study
Abstract
Objective:
Methods:
Results:
Conclusions:
Methods
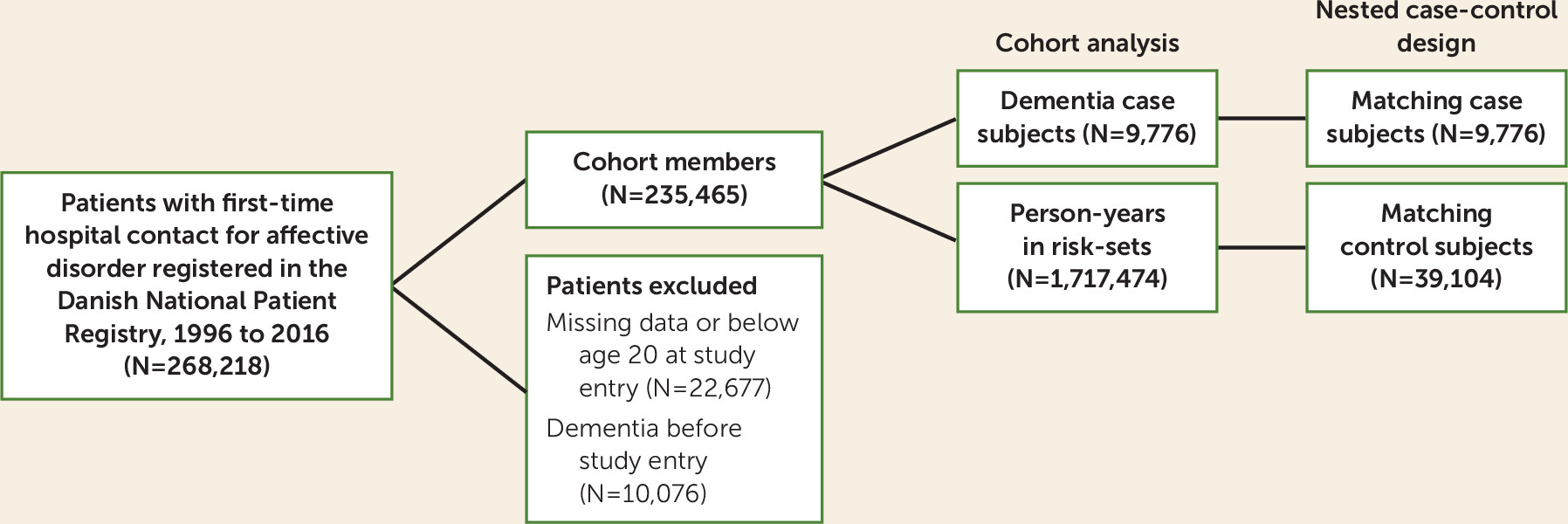
Study Population and Follow-Up
Outcome Definition
Exposure Definition (Benzodiazepines and Related Drugs)
Covariates
Statistical Analysis
Sensitivity Analysis
Results
| Total Cohort | Case Subjects With Dementia | Density-Sampled Control Subjects | ||||
|---|---|---|---|---|---|---|
| Characteristic | N | % | N | % | N | % |
| 235,465 | 9,776 | 39,104 | ||||
| Female | 144,002 | 61.2 | 6,414 | 65.6 | 26,883 | 68.8 |
| Marital status at baseline | ||||||
| Unmarried | 75,449 | 32.0 | 657 | 6.7 | 2,988 | 7.7 |
| Married | 92,504 | 39.3 | 4,106 | 42.1 | 17,075 | 43.8 |
| Divorced | 33,687 | 14.3 | 1,278 | 13.1 | 5169 | 13.3 |
| Widowed | 32,048 | 13.6 | 3,716 | 38.1 | 13,774 | 35.3 |
| Unknown | 1,777 | 0.8 | 19 | 0.2 | 58 | 0.4 |
| Educational level at baseline | ||||||
| Primary only (0–7 years of school) | 88,226 | 37.5 | 4,191 | 42.8 | 16,973 | 43.3 |
| Middle school | 88,724 | 37.7 | 2,450 | 25.1 | 10,281 | 26.3 |
| High school | 34,339 | 14.6 | 926 | 9.5 | 4,142 | 10.6 |
| Unknown | 24,176 | 10.3 | 2,209 | 22.6 | 7,708 | 19.7 |
| Depression subtype | ||||||
| Manic or bipolar disorder | 13,548 | 5.8 | 351 | 3.6 | 1,653 | 4.3 |
| Single or recurrent depression, mild | 31,898 | 13.6 | 1,374 | 14.1 | 5,631 | 14.4 |
| Single or recurrent depression, moderate | 65,771 | 27.9 | 2,206 | 22.6 | 9,357 | 23.9 |
| Single or recurrent depression, severe | 25,995 | 8.1 | 939 | 9.6 | 3,856 | 9.9 |
| Single or recurrent depression, other | 87,828 | 37.3 | 4,519 | 46.2 | 16,810 | 43.0 |
| Persistent or unspecified affective disorder | 10,425 | 4.4 | 387 | 4.0 | 1,797 | 4.6 |
| Psychotropic medication at baseline | ||||||
| Antipsychotic drugs | 54,051 | 24.0 | 2,479 | 25.4 | 9,870 | 25.3 |
| Antidepressant drugs | 175,307 | 74.5 | 7,258 | 74.2 | 29,620 | 75.8 |
| Comorbidity at baseline | ||||||
| Anxiety | 37,697 | 16.0 | 1,189 | 12.6 | 4,176 | 10.6 |
| Drug and alcohol abuse | 25,221 | 10.7 | 716 | 7.3 | 2,499 | 6.4 |
| Diabetes mellitus | 13,750 | 5.8 | 782 | 8.0 | 2,412 | 6.2 |
| Cardiovascular disease | 23,134 | 10.3 | 2,040 | 20.9 | 7,769 | 19.9 |
| Benzodiazepine and related drug useb | ||||||
| Any use | 179,249 | 76.1 | 7,942 | 81.2 | 33,666 | 86.1 |
| Any benzodiazepine use | 150,334 | 63.8 | 7,961 | 72.3 | 30,273 | 77.7 |
| Any Z-drug use | 128,683 | 54.7 | 5,170 | 52.9 | 23,691 | 60.6 |
| Any other anxiolytic use | 31,428 | 13.4 | 700 | 7.2 | 5,066 | 12.6 |
| Any long-acting drug use | 93,151 | 39.6 | 4,611 | 47.2 | 19,869 | 50.8 |
| Any short- and medium-acting drug use | 165,662 | 70.4 | 7,132 | 72.0 | 30,883 | 79.0 |
Any Use of Benzodiazepines, Related Drugs, and Dementia
| Variable | Age- and Gender-Adjusted Model | Fully Adjusted Modela | Fully Adjusted Model Plus Other Drugsb | |||
|---|---|---|---|---|---|---|
| Cohort study | ||||||
| Hazard Ratio | 95% CI | Hazard Ratio | 95% CI | Hazard Ratio | 95% CI | |
| Baseline | ||||||
| 0–2 years of follow-up, number of outcomes (N=5,085) | ||||||
| Any use | 0.69 | 0.66, 0.74 | 0.70 | 0.65, 0.75 | NA | |
| Benzodiazepine use | 0.72 | 0.67, 0.76 | 0.72 | 0.67, 0.76 | 0.74 | 0.69, 0.78 |
| Z-drug use | 0.77 | 0.73, 0.82 | 0.79 | 0.74, 0.84 | 0.82 | 0.78, 0.88 |
| Long-acting drug use | 0.80 | 0.75, 0.85 | 0.80 | 0.75, 0.84 | 0.82 | 0.77, 0.87 |
| Short- and medium-acting drug use | 0.73 | 0.69, 0.77 | 0.74 | 0.70, 0.78 | 0.75 | 0.71, 0.80 |
| Other anxiolytic use | 0.79 | 0.68, 0.91 | 0.85 | 0.73, 0.98 | 0.88 | 0.76, 1.02 |
| 2–20.1 years of follow-up, number of outcomes (N=4,691) | ||||||
| Any use | 0.99 | 0.92, 1.06 | 0.95 | 0.88, 1.02 | NA | |
| Benzodiazepine use | 1.01 | 0.95, 1.09 | 0.97 | 0.91, 1.04 | 0.97 | 0.92, 1.05 |
| Z-drug use | 0.95 | 0.89, 1.00 | 0.96 | 0.90, 1.02 | 0.96 | 0.90, 1.02 |
| Long-acting drug use | 1.03 | 0.98, 1.04 | 0.99 | 0.94, 1.05 | 0.99 | 0.93, 1.05 |
| Short- and medium-acting drug use | 0.98 | 0.92, 1.04 | 0.97 | 0.91, 1.03 | 0.97 | 0.91, 1.03 |
| Other anxiolytic use | 0.95 | 0.80, 1.10 | 1.00 | 0.85, 1.18 | 1.01 | 0.86, 1.18 |
| Nested case-control study | ||||||
| Odds Ratio | 95% CI | Odds Ratio | 95% CI | Odds Ratio | 95% CI | |
| 2 years before index date (1995) | ||||||
| Any use | 1.04 | 0.99, 1.10 | 0.97 | 0.92, 1.02 | NA | |
| Benzodiazepine use | 1.11 | 1.06, 1.16 | 1.03 | 0.98, 1.08 | 1.04 | 0.98, 1.09 |
| Z-drug use | 1.01 | 0.97, 1.06 | 0.96 | 0.91, 1.01 | 0.95 | 0.91, 1.00 |
| Long-acting drug use | 1.10 | 1.05, 1.15 | 1.02 | 0.97, 1.08 | 1.02 | 0.97, 1.02 |
| Short- and medium-acting drug use | 1.07 | 1.02, 1.12 | 1.01 | 0.96, 1.06 | 1.01 | 0.95, 1.06 |
| Other anxiolytic use | 0.98 | 0.88, 1.09 | 0.91 | 0.81, 1.01 | 0.91 | 0.81, 1.01 |
Cumulated Duration and Dose of Benzodiazepines and Dementia
| Measure | All Drugs | Benzodiazepines | Z-Drugs | Long-Acting Drugs | Short-Acting Drugs | Other Drugs | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Hazard ratio | 95% CI | Hazard ratio | 95% CI | Hazard ratio | 95% CI | Hazard ratio | 95% CI | Hazard ratio | 95% | Hazard ratio | 95% CI | |
| Cohort study (2–20.1 years of follow-up) | ||||||||||||
| Number of prescriptions | ||||||||||||
| None | 1 | 1 | 1 | 1 | 1 | 1 | ||||||
| 1–2 | 0.94 | 0.84, 1.04 | 1.00 | 0.92, 1.09 | 0.95 | 0.88, 1.03 | 0.99 | 0.91, 1.08 | 0.96 | 0.88, 1.04 | 0.94 | 0.77, 1.16 |
| 3–25 | 0.95 | 0.87, 1.03 | 0.97 | 0.90, 1.04 | 0.95 | 0.88, 1.02 | 0.98 | 0.92, 1.06 | 0.98 | 0.91, 1.05 | 1.13 | 0.86, 1.47 |
| 26 (maximum) | 0.95 | 0.87, 1.04 | 0.95 | 0.87, 1.04 | 0.98 | 0.87, 1.09 | 1.01 | 0.91, 1.11 | 0.98 | 0.89, 1.07 | 0.96 | 0.43, 2.15 |
| Total defined daily dose | ||||||||||||
| None | 1 | 1 | 1 | 1 | 1 | 1 | ||||||
| Lowest third | 0.99 | 0.84, 1.03 | 0.99 | 0.90, 1.08 | 0.96 | 0.87, 1.05 | 0.95 | 0.86, 1.03 | 0.99 | 0.90, 1.08 | 0.97 | 0.76, 1.24 |
| Middle | 0.95 | 0.86, 1.03 | 0.97 | 0.89, 1.05 | 0.92 | 0.84, 1.00 | 1.02 | 0.93, 1.11 | 0.94 | 0.84, 1.02 | 1.02 | 0.78, 1.36 |
| Highest third | 0.94 | 0.86, 1.03 | 0.97 | 0.89, 1.04 | 0.98 | 0.90, 1.00 | 1.00 | 0.92, 1.08 | 0.98 | 0.89, 1.05 | 1.00 | 0.72, 1.40 |
| Odds ratio | 95% CI | Odds ratio | 95% CI | Odds ratio | 95% CI | Odds ratio | 95% CI | Odds ratio | 95% CI | Odds ratio | 95% CI | |
| Nested case-control study (2 years before index date, 1995) | ||||||||||||
| Number of prescriptions | ||||||||||||
| None | 1 | 1 | 1 | 1 | 1 | 1 | ||||||
| 1–2 | 1.23 | 1.12, 1.35 | 1.16 | 1.06, 1.26 | 1.11 | 1.03, 1.20 | 1.07 | 0.99, 1.16 | 1.20 | 1.10, 1.31 | 1.09 | (0.94–1.26) |
| 3–25 | 1.01 | 0.95, 1.07 | 1.06 | 1.00, 1.13 | 0.98 | 0.92, 1.04 | 1.08 | 1.01, 1.15 | 1.05 | 0.99, 1.11 | 0.88 | (0.74–1.05) |
| 26 (maximum) | 0.87 | 0.82, 0.93 | 0.94 | 0.88, 1.00 | 0.84 | 0.79, 0.90 | 0.91 | 0.84, 0.97 | 0.90 | 0.84, 0.96 | 0.51 | 0.37, 0.69 |
| Total defined daily dose | ||||||||||||
| None | 1 | 1 | 1 | 1 | 1 | 1 | ||||||
| Lowest third | 1.08 | 1.01, 1.15 | 1.08 | 1.01, 1.15 | 1.25 | 1.17, 1.35 | 1.05 | 0.95, –1.13 | 1.11 | (1.05–1.19) | 1.15 | 0.97, 1.36 |
| Middle | 0.99 | 0.92, 1.05 | 1.07 | 1.00, 1.14 | 1.07 | 0.98, 1.14 | 1.10 | 1.02, 1.12 | 0.97 | (0.87–1.10) | 0.86 | 0.71, 1.03 |
| Highest third | 0.83 | 0.77, 0.88 | 0.91 | 0.85, 0.91 | 0.92 | 0.86, 1.00 | 0.92 | 0.85, 0.98 | 0.86 | 0.80, 0.92 | 0.73 | 0.66, 0.88 |
Sensitivity Analyses
Discussion
Comparison With Other Studies
Strengths and Limitations
Explanations and Implications
Supplementary Material
- View/Download
- 314.23 KB
References
Information & Authors
Information
Published In
History
Keywords
Authors
Competing Interests
Funding Information
Metrics & Citations
Metrics
Citations
Export Citations
If you have the appropriate software installed, you can download article citation data to the citation manager of your choice. Simply select your manager software from the list below and click Download.
For more information or tips please see 'Downloading to a citation manager' in the Help menu.
View Options
View options
PDF/EPUB
View PDF/EPUBLogin options
Already a subscriber? Access your subscription through your login credentials or your institution for full access to this article.
Personal login Institutional Login Open Athens loginNot a subscriber?
PsychiatryOnline subscription options offer access to the DSM-5-TR® library, books, journals, CME, and patient resources. This all-in-one virtual library provides psychiatrists and mental health professionals with key resources for diagnosis, treatment, research, and professional development.
Need more help? PsychiatryOnline Customer Service may be reached by emailing [email protected] or by calling 800-368-5777 (in the U.S.) or 703-907-7322 (outside the U.S.).

