Repetitive transcranial magnetic stimulation (rTMS) delivered to the left dorsolateral prefrontal cortex (DLPFC) is a noninvasive brain stimulation technique approved by the U.S. Food and Drug Administration (FDA) for treatment-resistant depression (
4). rTMS involves passing an electrical current through a magnetic coil placed superficial to the scalp, producing a high-intensity magnetic field that passes through the scalp, skull, and meninges to excite neuronal tissue (
5). Repeated high-frequency excitation of the same brain region results in strengthening of synapses through a process known as long-term potentiation (
6,
7), causing changes in functional connectivity (
6,
8). The mechanism of rTMS on the core depressive symptoms is hypothesized to be mediated in part through indirect inhibitory functional connectivity from the left DLPFC to the subgenual anterior cingulate cortex (sgACC) (
8–
11).
A more efficient form of rTMS, known as intermittent theta-burst stimulation (iTBS), recently approved by the FDA, has significantly shortened the duration of rTMS treatment sessions from 37 minutes to 3 minutes (
12) and produces equivalent antidepressant responses (
13,
14). FDA-approved rTMS and iTBS courses involve daily stimulation sessions (600 iTBS pulses) for 6 weeks, and one trial has demonstrated remission in 32% of patients and response in 49% (
13). Studies suggest that the efficacy of iTBS might be improved by accelerated, spaced delivery of stimulation sessions (
15–
18), higher overall pulse doses (
19–
21), and individualized targeting (
8,
22).
We investigated the safety, tolerability, and preliminary efficacy of an accelerated high-dose iTBS protocol using functional connectivity MRI (fcMRI)–guided targeting. This protocol involved 5 consecutive days (Monday through Friday) of 10 iTBS sessions per day (1,800 pulses per session, 50-minute intersession intervals) delivered to the region of the left DLPFC that is most functionally anticorrelated with the sgACC in each participant (
23). This protocol was termed Stanford Accelerated Intelligent Neuromodulation Therapy (SAINT), to distinguish it from other attempts at accelerating TMS protocols without individualized targeting, 50-minute intersession intervals, or high pulse dose (
24,
25). Our initial investigation of SAINT (
23) demonstrated efficacy in a small cohort of participants with severe and treatment-refractory depression (these participants are not included in the present study). The study we describe here builds on our initial report by testing SAINT in a larger and more generalizable cohort of participants with treatment-resistant depression to examine the feasibility, safety, and preliminary efficacy of this approach.
Methods
Participants
Participants were required to be currently experiencing a nonpsychotic major depressive episode as part of either major depressive disorder or bipolar II disorder as defined by DSM-5 criteria and to have not responded to at least one antidepressant medication. At the time of screening, participants were required to have a 17-item Hamilton Depression Rating Scale (HAM-D) score ≥20, a negative urine drug screen, and a negative urine pregnancy test if female. Participants were excluded if they had any contraindications to rTMS, such as a history of seizures, metallic implants in the head, cardiac pacemakers, or a neurological disorder. Participants were recruited through the Depression Research Clinic at Stanford University, study advertisements, and clinic referrals.
Twenty-three participants (ages 19–78, 13 female) were recruited for this study. One participant was screened out after enrollment for having a very high motor threshold (>90% machine output) and one participant with a history of multiple prior therapeutic intolerances (anxiety leading to early discontinuation of intravenous ketamine infusions and conventional rTMS) dropped out after the first day of stimulation because of anxiety. This resulted in a final sample of 21 participants (ages 19–78, 12 female). Nineteen participants had a diagnosis of major depressive disorder, and two had a diagnosis of bipolar II disorder currently in a depressive episode (>1 year).
Table 1 summarizes participants’ demographic characteristics and treatment history. Participants were required to maintain their antidepressant regimen throughout study enrollment (for medications taken during enrollment, see Table S1 in the
online supplement). Six participants were retreated when they no longer met the criterion for remission and again met the study entry criterion (HAM-D score ≥20). Mean time between treatments was 20.5 weeks (SD=6.6).
All research procedures were conducted in accordance with the ethical standards outlined in the Declaration of Helsinki. The study was approved by the Stanford University Institutional Review Board, and all participants provided written informed consent before taking part in any study procedures.
Functional MRI
Before the stimulation course, each participant had both structural MRI and resting-state functional MRI (fMRI) scans. All MRI scans were acquired using a 3-T GE Discovery MR750 scanner with a 32-channel imaging coil at the Center for Cognitive and Neurobiological Imaging at Stanford University, using a simultaneous multi-slice (SMS) imaging sequence with an acceleration factor of 3 (SMS=3), which collects three equally spaced axial slices simultaneously. A total of 29 sets of three slices, for a total of 87 slices, were collected within each repetition time of 2 seconds. During the 8-minute resting-state scans, participants were instructed to let their minds wander, avoid repetitive thoughts, keep their eyes open, and keep their attention focused on a central fixation point.
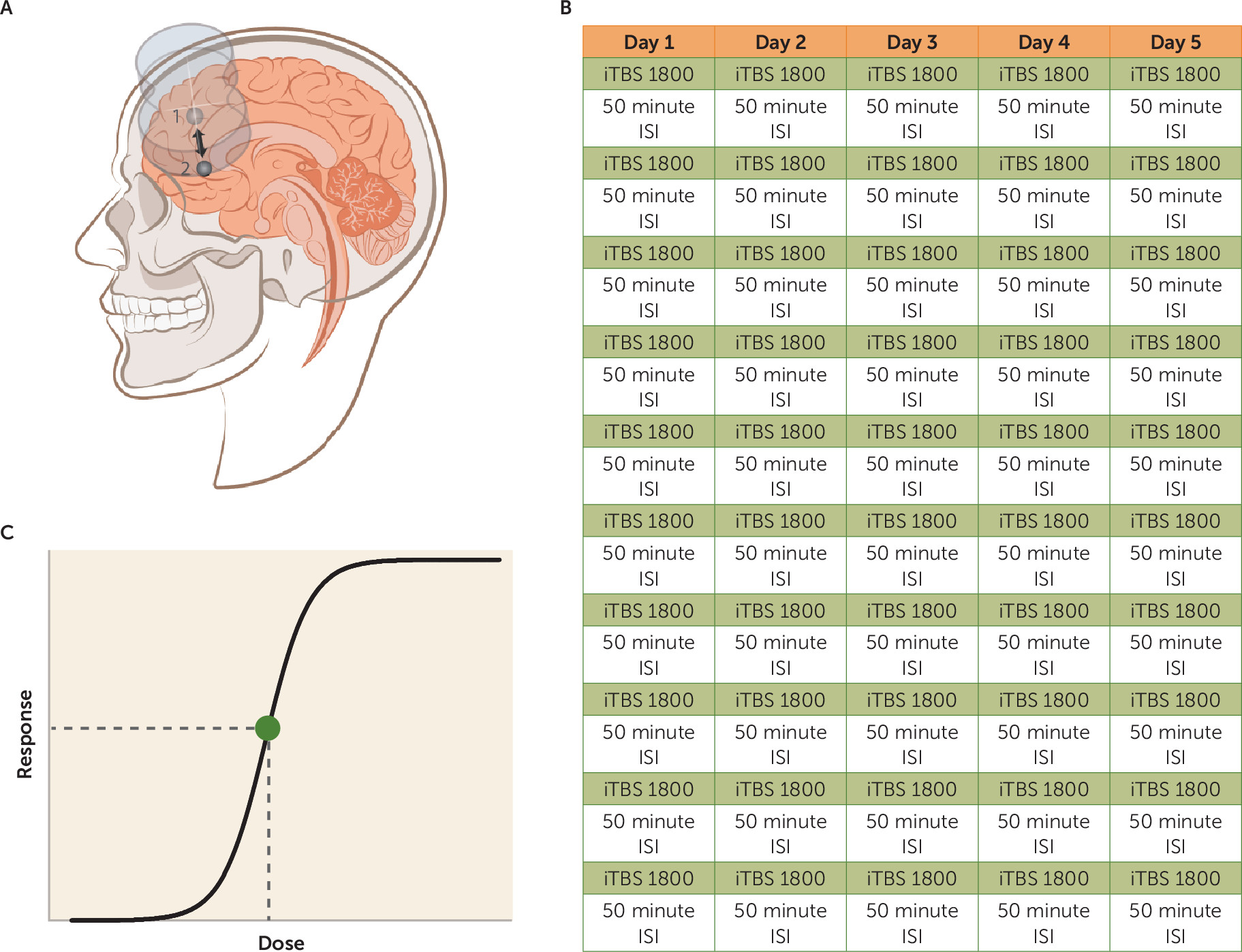
Stanford Accelerated Intelligent Neuromodulation Therapy
A MagVenture MagPro X100 (MagVenture A/S, Farum, Denmark) system was used to deliver sessions of iTBS; 60 cycles of 10 bursts of three pulses at 50 Hz were delivered in 2-second trains (5 Hz) with an 8-second intertrain interval. Stimulation sessions were delivered hourly (
15–
17). Ten sessions were applied per day (18,000 pulses/day) for 5 consecutive days (90,000 pulses in total). Stimulation was delivered at 90% resting motor threshold (rMT) (
13,
26,
27). A depth correction was applied (
28) to consistently achieve 90% rMT at the depth of the functional target. For safety, stimulation was never delivered above 120% rMT (
13). The Localite Neuronavigation System (Localite GmbH, Sankt Augustin, Germany) was used to position the TMS coil over the individualized stimulation target every session. See
Figure 1 for differences between SAINT and the FDA-approved iTBS protocol. In between treatments, participants were seated in a reserved waiting area, which was not occupied with study staff or other patients. This was done both to limit the interaction time with study staff and to prevent a group effect. All iTBS sessions were delivered in the Department of Psychiatry and Behavioral Sciences at Stanford University on an outpatient basis.
Clinical Assessments
Before and after SAINT, depressive symptoms and suicidal ideation were assessed using clinical and self-report assessments (HAM-D, Montgomery-Åsberg Depression Rating Scale [MADRS], Columbia-Suicide Severity Rating Scale [C-SSRS; suicidal ideation subscale], and Beck Depression Inventory–II [BDI-II]). At the end of each day’s 10 stimulation sessions, depressive symptoms were assessed using the 6-item HAM-D. The Young Mania Rating Scale was completed daily to assess for hypomania (
26).
A neuropsychological test battery was administered before and after SAINT to capture any neurocognitive side effects. The Hopkins Verbal Learning Test–Revised (
27), the Brief Visuospatial Memory Test–Revised (
29), subtests from the Wechsler Adult Intelligence Scale, 4th ed., and several tests from the Delis Kaplan Executive Function System (
30) were used. See the
online supplement for detailed information about the neuropsychological test battery.
fMRI Analysis for Target Generation
Personalized left DLPFC targets were generated for each participant, using the baseline resting-state scan. All analyses were conducted in the participant’s own brain space. Resting-state scans were preprocessed according to typical methods using the Statistical Parametric Mapping program (SPM12). The resting-state scans were motion corrected and resliced. T1-weighted structural scans were then co-registered with the resting-state scans. Next, the estimation parameters to warp the T1-weighted structural image into Montreal Neurological Institute (MNI) space were calculated using SPM segmentations based on tissue probability maps. These normalization parameters were inverted and applied to MNI space regions of interest for the left DLPFC (Brodmann’s area [BA] 46) and the sgACC (BA25) to map these regions of interest onto the individual participant’s brain. The participant-space regions of interest were then resliced, smoothed, and binarized to match the dimensions of the resting-state scans. See the online supplement for more detailed information regarding fMRI processing.
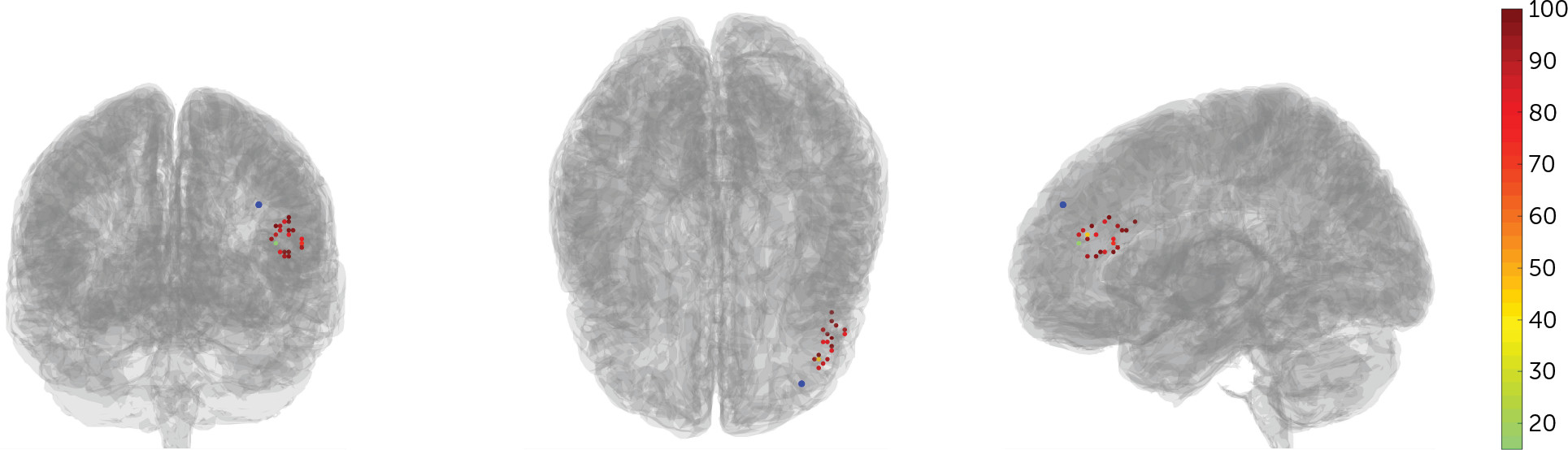
The participant-space regions of interest for the left DLPFC formed the search area for TMS coil placement. Two separate algorithms were used to determine coil placement. The first algorithm sorted each of the left DLPFC and left and right sgACC voxels into functional subunits using a hierarchical agglomerative clustering algorithm. The voxel time series that most accurately reflected the median time series was then created for each functional subunit, and the correlation coefficients were calculated between all selected time series extracted from all functional subunits of the left DLPFC and sgACC. Median time series were used rather than mean time series, because median values are not susceptible to high signal outliers. The second algorithm determined the optimal left DLPFC subunit to target, based on three factors: the net correlation/anticorrelation of the left DLPFC subunit with sgACC subunits, the size of the subunit, and the spatial concentration of the subunit. See the
online supplement for more details on these algorithms. Three-dimensional maps of the whole brain correlation coefficient of the selected left DLPFC subunit were then created and used to target the coil placement, using the Localite TMS Navigation software program. See
Figure 2 for the individual target locations.
Clinical Outcome Analysis
All statistical analyses were conducted using SPSS, version 25 (IBM, Armonk, N.Y.). The level of statistical significance was set at p=0.05. Missing data were not imputed. Statistical analyses were planned independently by two authors (B.S.B. and B.J.) and reviewed by two authors (A.F.S. and N.R.W.).
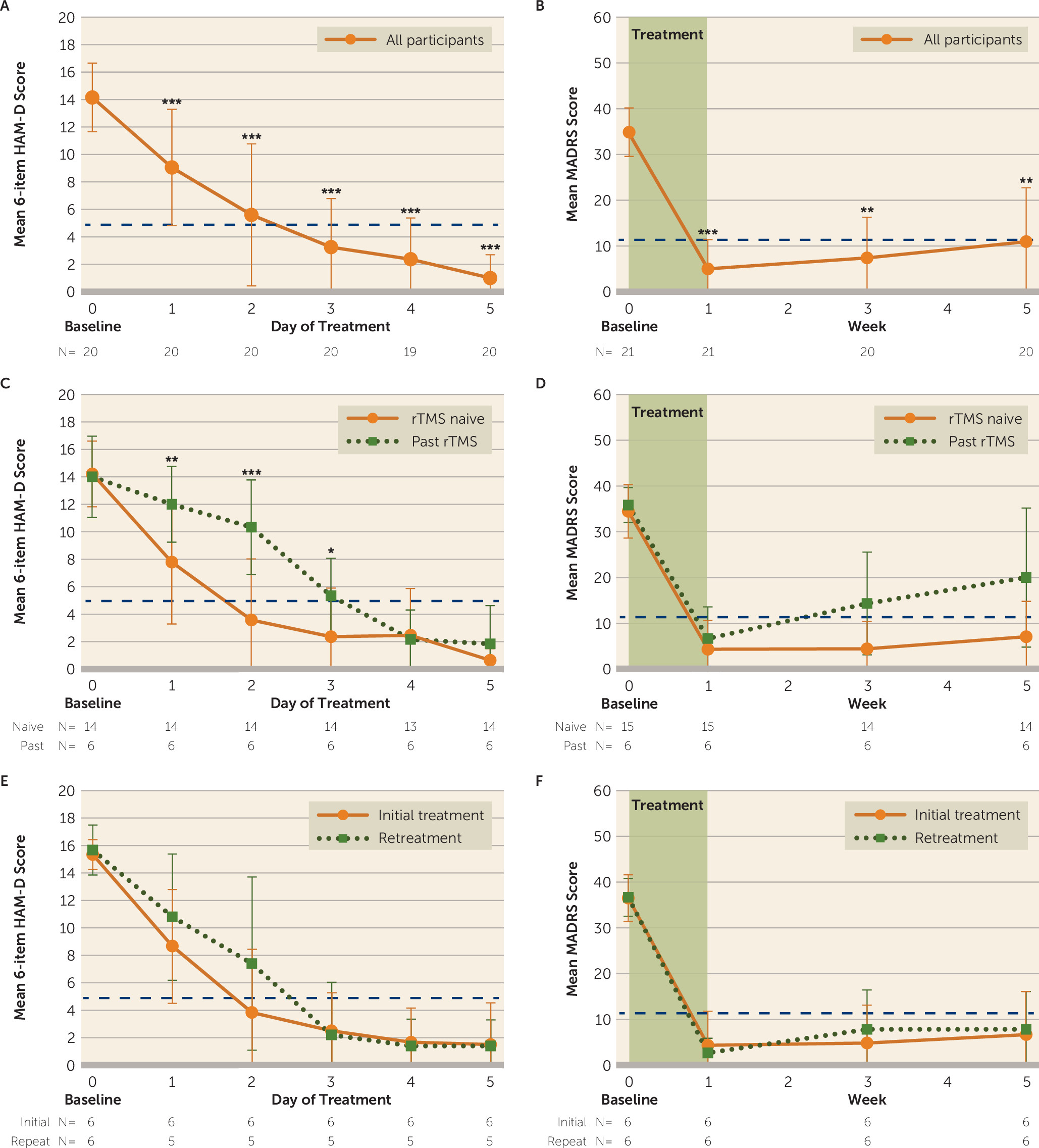
Our primary outcome measure was change in MADRS score from baseline to immediately after SAINT, and MADRS scores were used to calculate response and remission rates. Reductions in scores on the 17-item and 6-item HAM-D and the BDI-II were used as secondary outcome measures of depression severity. Response was defined as a reduction ≥50% on these scales. Remission was defined as score <11 on the MADRS (
31), a score <8 on the 17-item HAM-D (
32), a score <5 on the 6-item HAM-D (
33), and a score <13 on the BDI-II (
34). A floor effect of SAINT treatment was observed across all scales, and initial linear mixed models produced residuals that were not normally distributed, as determined by the Shapiro-Wilk test. Thus, changes in scores on the MADRS, the 6- and 17-item HAM-D, and the BDI-II were assessed with generalized linear mixed models that used a compound symmetry covariance structure, Satterthwaite approximation of degrees of freedom, and robust estimation of coefficients to handle violations of model assumptions. Fixed effects of time, treatment course (initial versus retreatment), and a treatment history of nonresponse to conventional rTMS and their interactions were assessed. All post hoc pairwise comparisons were Bonferroni corrected.
Daily 6-item HAM-D scores were used to calculate the number of days of stimulation required to reach the response criterion (a reduction ≥50% from baseline) and the remission criterion (a score <5). Kaplan-Meier survival analysis using the Breslow test of equality of survival distributions was used to determine whether there were significant differences in the number of days to reach response and remission criteria for participants who had a history of nonresponse to conventional rTMS compared with those who did not.
Suicidality was assessed using the suicidal ideation subscale of the C-SSRS, item 3 of the 17-item HAM-D, and item 10 of the MADRS. Response was defined as a reduction ≥50% in these scores from baseline, and remission was defined as a score of 0. Response was calculated only if the baseline score was >0. Scores were ordinal, and changes in scores were assessed with generalized linear models with a multinomial link, compound symmetry covariance structure, Satterthwaite approximation of degrees of freedom, and robust estimation of coefficients to handle violations of model assumptions.
Scores on the neuropsychological tests before and after SAINT were compared using paired t tests. The data for total score on the Hopkins Verbal Learning Test–Revised, score on the delayed recall trial from the Brief Visuospatial Memory Test–Revised, and the number of rule violations on the tower test from the Delis Kaplan Executive Function System violated the assumption of normality, so Wilcoxon signed-rank tests were used to evaluate SAINT-induced changes in performance on these three measures. Neuropsychological test data were available for 17 participants.
Discussion
The aim of this study was to examine the safety, feasibility, and preliminary efficacy of an accelerated, high-dose, fcMRI-guided iTBS treatment protocol (SAINT) for treatment-resistant depression. We found that SAINT significantly reduced depressive symptoms and suicidal ideation in patients with treatment-resistant depression within 5 days, without negative cognitive side effects. The remission rate we observed is higher than reported open-label remission rates for standard FDA-approved rTMS protocols (37%) (
13,
35,
36), ECT (∼48%) (
37), and ketamine (31%) (
38) for treating treatment-resistant depression (see Table S5 in the
online supplement). The difference between the observed remission rate for SAINT and remission rates reported for standard rTMS protocols may be due to the spaced stimulation sessions, accelerated delivery, high pulse dose, individualized targeting, higher sham effect, or a combination of these. The individual contribution of each of these elements cannot be determined from this study, as they were not investigated separately. Double-blinded trials will be needed to determine the contribution of the sham effect.
The 50-minute spacing in our SAINT protocol complements evidence from basic neuroscience research and human physiology data, which suggest that multiple, spaced daily iTBS sessions have an enhanced effect compared with the same number of single daily sessions (
15–
17,
39–
42). Multiple, spaced sessions have also been shown to produce accumulating nonlinear improvements in clinical symptoms (
41,
43). The duration of intersession intervals is likely to be important, as stimulation sessions with intersession intervals of 50–90 minutes have been shown to have a cumulative effect on synaptic strengthening, whereas sessions with intersession intervals of 40 minutes or less do not show this cumulative effect (
15–
17,
44). Similarly, some studies have shown that two theta-burst stimulation sessions delivered to the motor cortex 15 minutes apart do not increase cortical excitability compared with a single session (
42,
45). Finally, left DLPFC activity has been shown to be correlated with sgACC activation 10 minutes after rTMS; this correlation was reduced 27 minutes after rTMS, and the correlation between these regions reached its nadir 45 minutes after rTMS (
10), the hypothetically optimal time for stimulation. Taken together, these data could explain the limited response rate (39%) of a previously reported accelerated iTBS stimulation protocol for depression (
24,
25), which used an intersession interval of 15 minutes, with 20 sessions delivered over 4 days. In comparison, a previous study utilizing intervals of 1 hour between sessions (
46) obtained a similar response rate (43%) after only 15 sessions of conventional rTMS treatment over 2 days. However, the study with 15-minute intersession intervals was a randomized controlled trial, so the response rates cannot be directly compared with ours in the present study.
The functional connectivity–guided targeting method used in our study may have contributed to the 90% remission rate we observed. The left DLPFC is a large brain area that consists of several subregions, some of which are correlated and some anticorrelated with sgACC activity (
47). Recent work suggests that these correlated and anticorrelated subregions are part of different affective circuits; stimulating a subregion of the left DLPFC that is anticorrelated with the sgACC reduces melancholic symptoms, resulting in lower MADRS score (
48,
49). In contrast, targeting a left DLPFC subregion correlated with the sgACC reduces anxiosomatic symptoms and results in reduced anxiety ratings (
48). The anxiosomatic target is within range of the 5-cm rule coil position, whereas the melancholic target is more anterior and lateral within BA46 (
48). Our targeting approach was in broad agreement with the target identified to produce maximum clinical change in previous studies (
22), which has been demonstrated to be centered in BA46 (
8). However, we further extend this strategy by parsing the BA46 region into functional subunits based on the degree of correlation/anticorrelation with the functional subunits of the sgACC (
23).
The individualization of our targeting approach may also be important; a trial in healthy individuals showed that stimulating the left DLPFC using personalized functional connectivity–guided targeting induced the desired change in functional connectivity between the left DLPFC and the sgACC (
10). A recent interleaved TMS-fMRI study showed that when using individualized functional connectivity–guided targeting, stimulation propagated from the left DLPFC to the sgACC in all participants (
50). In comparison, a separate study defined the left DLPFC anatomically (border of BA9/BA46) and stimulation propagated to the sgACC in only 44% of participants (
51). By stimulating the subregion of the left DLPFC that is most anticorrelated with the sgACC in each individual, we may have reduced variability in signal propagation to the intended brain target as well as improved treatment efficacy of the core depressive symptoms (
8,
48). Additional studies are needed to determine how important individualized fcMRI-guided targeting methods are relative to fcMRI-guided methods based on group-average fMRI data. Clinical studies are needed to directly compare remission rates following iTBS/rTMS protocols with and without individualized targeting.
Our SAINT protocol administered five times the overall pulse dose of the FDA-approved iTBS protocol, as well as a higher density of stimulation (90,000 pulses in 5 days, compared with the standard 18,000 iTBS pulses in 6 weeks [
13]). Previous studies found that 61% of individuals who do not respond to an rTMS treatment course responded with additional rTMS treatment sessions (
20) and that higher overall pulse doses are associated with higher efficacy (
52,
53). A recent report demonstrated nonasymptotic negative linear relationships between the number of rTMS treatments and depression symptom scores (
54). This suggests that higher overall pulse doses might further reduce depression symptoms. The apparent need for higher pulse dose is consistent with deep brain stimulation in other neuropsychiatric disorders, where ∼500,000 pulses of stimulation are delivered each day (
55). Our SAINT protocol applies an amount of stimulation equivalent to a 6-week standard iTBS treatment protocol (18,000 pulses) each day of stimulation (
13). Thirty percent of participants in our study met response criteria after the first day of stimulation (N=6/20; daily 6-item HAM-D scores missing for one participant), which is equivalent to response rates for iTBS/rTMS for this treatment resistance level (
56–
60). None of the nonresponders to prior rTMS in our study responded after a single day of SAINT (see
Figure 3), whereas 83% of these prior rTMS nonresponders did respond by the end of the 5-day protocol. Our study administered the highest number of TMS pulses per day and the highest overall TMS pulse dose of any study we are aware of. It is possible that standard FDA-approved rTMS protocols may benefit from higher overall pulse doses.
Prior rTMS nonresponders in our study required more stimulation sessions to induce a clinically significant response. It is possible that depressed individuals with a higher degree of treatment resistance display neuroplasticity impairments (
61). Thus, highly treatment-resistant individuals may require a higher pulse dose to induce an antidepressant response, and individuals with the highest degree of treatment resistance may require maintenance iTBS therapy (
62) or even an implanted cortical stimulator (
63,
64) to induce and sustain antidepressant response (
20).
Our study has several limitations, including a small sample size and an open-label design. The small sample size in our study means that the treatment effect may have been influenced by unknown factors related to sampling biases, which emphasizes the need for larger double-blinded sham-controlled trials. Moreover, without a sham-control group we cannot rule out the possibility that our results are primarily due to sham effect; a multicenter rTMS trial in veterans with treatment-resistant depression found equivalent remission rates in the active (40.7%) and sham (37.4%) groups (
65). The sham effect for SAINT may be particularly high because of the frequency of the treatment sessions (10 per day) and the perceived novelty of the method. However, a previous study found that individuals with high treatment refractoriness, like many of the participants in this study, showed no sham response to iTBS sessions of 1,800 pulses (
13,
66), and an observational study monitoring 124 individuals with treatment-resistant depression receiving treatment as usual (medications, psychotherapy, and ECT) showed a 3.6% remission rate after 1 year of treatment (
67), demonstrating the low incidence of spontaneous remission in this population. The remission rate observed in this study is higher than rates reported in previous open-label interventions for treatment-resistant depression (see Table S5 in the
online supplement). Double-blinded sham-controlled trials are needed to determine the contribution of the sham effect to the high remission rate observed for SAINT.
Further methodological uncertainties include stimulation of a single brain region (
68), fixed stimulation frequencies (
54,
69), fixed intersession intervals (
69,
70), and the lack of state-dependent stimulation (
71). Individualized stimulation frequencies may result in quicker and more durable responses (
69,
72), and different cortical excitability profiles may require different intersession intervals (
70,
73). Finally, recent studies have shown that applying stimulation in particular brain states using real-time EEG-triggered TMS can increase cortical responses to stimulation (
71).
In conclusion, SAINT, our high-dose, accelerated, fcMRI-guided iTBS protocol, is preliminarily safe, feasible, and associated with a high rate of remission from depression. The potential efficacy of SAINT in treating suicidal ideation and the short duration of the protocol suggest that SAINT could provide a means of rapidly ensuring the safety of suicidal patients. However, larger, double-blinded, sham-controlled trials are required to confirm the results from this initial study. We remain cautious in our enthusiasm about the present results, as this is an open-label study with all the shortcomings associated with uncontrolled studies.