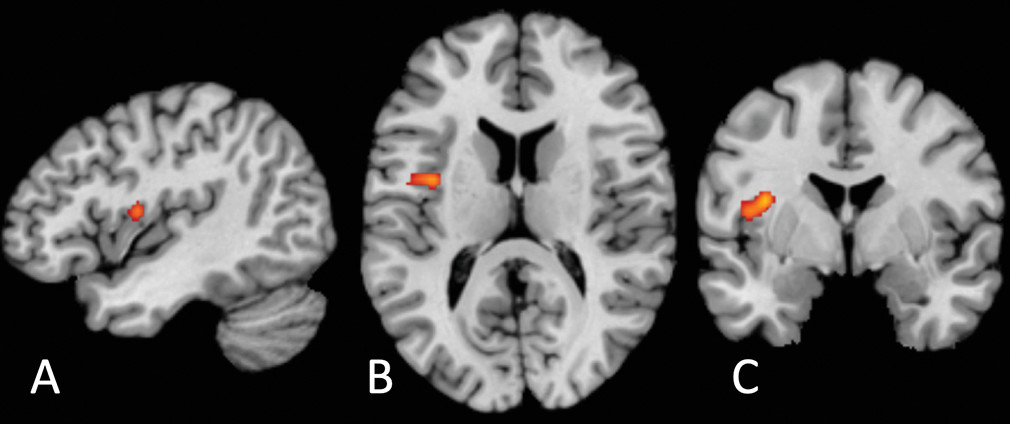
The locus of transdiagnostic differential neural activation we report is located on the precentral gyrus of the mid-insula (
49). Nonhuman primate studies have established that the insula comprises three cytoarchitectonic subregions: a ventral-anterior agranular area, a mid-insular dysgranular zone, and a dorsal-posterior granular area (
27,
50,
51). Human in vivo probabilistic tractography (
52) and anatomical tracing studies in macaques (
27) have revealed a graduated pattern of connectivity: agranular anterior insula projects to inferior frontal regions and some anterior temporal areas, while granular posterior insula projects primarily to posterior superior and middle temporal areas (
52). In contrast, the mid-insular dysgranular zone possesses a hybrid connectivity pattern: the precentral insular gyrus projects to both frontal and temporal regions (
27,
52), and its afferent inputs likewise originate from a hybrid of regions projecting to anterior and posterior insula (
53). Moreover, while anterior and posterior insula show dense within-subregion connectivity (
52), the mid-insula region identified here, in particular the precentral insular gyrus, projects to (
27,
52) and receives input from (
53) both anterior and posterior insula. This makes the mid-insula well placed to serve as an intermediary between the processing of sensory representation of bodily state in the posterior insula (
31) and the abstract representation of affective state in the anterior insula (
34), where the latter, as demonstrated empirically in our conjunction analysis, appears to be spatially distinct from our identified region of interoceptive dysfunction (
31,
34,
38).
The dorsal mid-insula could represent a common locus of disruption emerging from other (domain-specific or pathology-specific) interoceptive changes. Some pathologies may originate from a primary dysfunction in interosensory signaling (e.g., an increased sensitivity to interoceptive stimuli, resulting in a higher weighting of prediction errors [
54]), while others may stem from increased precision of prediction models, at the expense of prediction error signals (thought to be encoded by neuromodulators [
55] or local GABAergic mechanisms [
56]). We speculate that the unique hybrid architecture of the dysgranular dorsal mid-insula makes it a possible anatomical candidate for encoding of interoceptive prediction errors. This hypothesis requires testing in future studies employing tasks that manipulate the expectancy of interoceptive associations and fit learning models to trial-by-trial prediction errors (a paradigm commonly employed in exteroceptive predictive processing studies, e.g.,
57). This approach could delineate the putative role of the dysgranular mid-insula in interoceptive predictive processing in psychiatric populations.
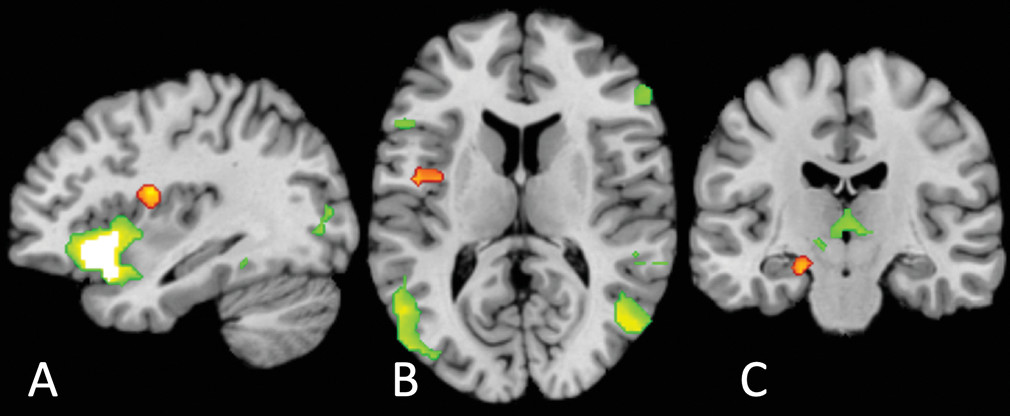
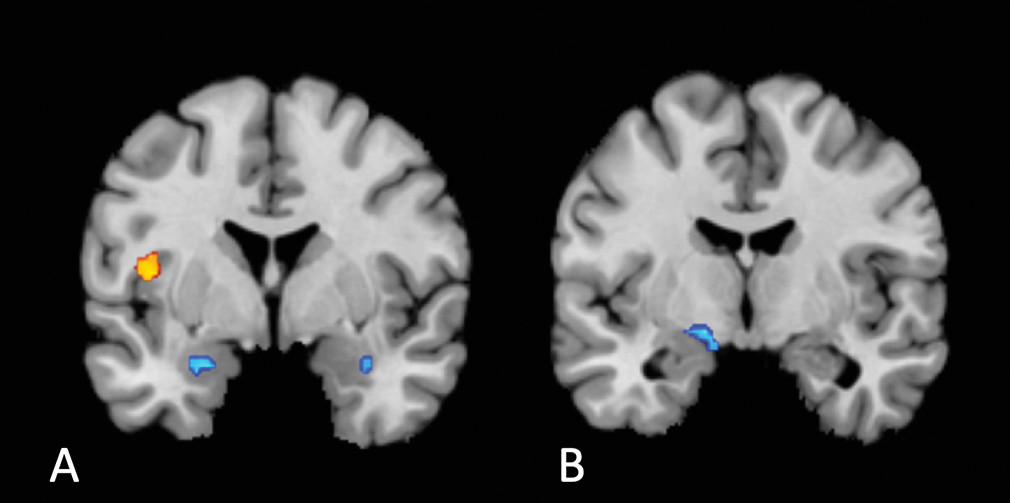
Two aspects of the location of our common cluster are surprising. The first is its laterality: previous findings strongly suggest a right lateralization of interoception (
1). While our uncorrected results do include multiple smaller clusters in the right insula and claustrum, it is possible that this general lateralization of function is not reflected in right lateralization of disrupted function or that low power in the included studies resulted in weaker clustering on the right. In addition, it is surprising that our result does not overlap with the extensive affect network, given the strong overlap of interoceptive-frontotemporal regions subserving interoception and emotion in general (
32,
58). We suggest that this originates from our locus occurring lower in the interoceptive hierarchy than might have been expected: the affect circuitry results clearly show a large, highly significant cluster in the anterior insula but not the mid- or posterior insula.
Limitations and Future Directions
The locus of disruption we report appears common across several disorders in our analysis: patients with unipolar and bipolar depression, anxiety, anorexia, and schizophrenia all fell within the cluster identified. Nevertheless, there may still exist anatomically distinct processing alterations in discrete disorders or specific transdiagnostic dimensions. The current literature does not contain sufficient data to provide the statistical power necessary for robust disorder-specific subanalyses. In addition, given the nature of our meta-analytic approach, it was not possible to conduct meta-regression analyses to examine the influence of sex and age on interoceptive differences in psychiatric disorders (
59). This is because ALE tests for convergence of activation between studies, with no incorporation of different effect sizes (e.g., fMRI Z
-score), a prerequisite for meta-regression (
59). The exploration of latent variables that underlie our activation differences is an important avenue for future research. Although we conducted exploratory subgroup analyses of different symptom domains (see the Materials section in the
online supplement), these subgroup analyses (as well as any future subgroup analyses testing effects of gender or age) will require a larger number of included studies to achieve sufficient statistical power and therefore remain a key question for future studies.
In addition, conclusions from the existing literature are limited by the fact that certain interoceptive domains are measured significantly more in some disorders than in others: disorder-specific results in the literature may partially be driven by task differences. By aggregating across studies that measure different interoceptive functions (nociception, respiration, cardiac attention, sensory touch, etc.), our meta-analysis is unable to disentangle the contribution of these task differences themselves. We did not have the statistical power to analyze convergent disrupted activation within specific interoceptive domains, despite the strong likelihood that different interoceptive channels are not necessarily integrated (
42,
60). Therefore, we were unable to identify how the functional role of the insula (or any other region) in psychiatric disorders might differ across different features of interoception. Our result represents only a transdiagnostic, cross-domain alteration; in reality, transdiagnostic alterations could be observed within specific interoceptive domains. For example, in the respiratory domain, animal work has identified acid-sensing ion channels in the basolateral amygdala and bed nucleus of the stria terminalis that drive carbon-dioxide-evoked fear behavior; genetic variations in the sensitivity of these ion channels in humans relate to susceptibility to carbon dioxide challenge and, potentially, panic attacks (reviewed in reference
61).
Future neuroimaging work could better elucidate how interoceptive processing in the mid-insula (and elsewhere in interoceptive brain circuitry) might differ between particular (clusters of) disorders or symptom dimensions, consistent with recent dimensional neuroimaging approaches (
62,
63). This would enable future neurocognitive treatment development focused on interoceptive loci and designed to modulate disrupted interoceptive processing, similar to the application of novel brain stimulation interventions to target particular transdiagnostic neurocognitive mechanisms (
64,
65). This potential is further supported by our finding that neither antidepressant medication nor psychological therapy appears to evoke activation changes in this mid-insular region, albeit using mostly noninteroceptive tasks. This highlights the need for treatment studies employing interoceptive probes. However, existing interoceptive measures are limited in many respects: tasks often employ explicit interoceptive manipulations (e.g.,
66), while interoception itself is usually unconscious (
67), requiring invasive perturbation approaches (e.g.,
68), and the timescales of interoceptive signals range vastly between systems (e.g., respiratory systems compared with gastric systems). Sophisticated computational approaches go some way toward parameterizing disruptions in interoceptive signaling (
19) but are still subject to many of the above limitations and also usually require extremely lengthy procedures to estimate computational parameters.
Interoceptive processing is multidimensional. A recent consensus definition identified eight aspects of interoception: interoceptive attention (observing one’s internal bodily sensations), detection (reporting the presence or absence of a consciously reported sensation), magnitude (perceived intensity of a sensation), discrimination (localizing a sensation to a particular interoceptive channel), accuracy (how correct is one’s monitoring of sensations), insight (metacognitive evaluation of one’s interoceptive performance, i.e., the correspondence between accuracy and confidence), sensibility (a trait measure, the self-assessed tendency to focus on interoceptive stimuli), and self-report (assessed with psychometric questionnaires that can be state or trait assessments). These dimensions share some common features but likely have relatively distinct neural mappings (
2,
69). All studies in this meta-analysis probed brain activation either by using bottom-up perturbations of sensory signals (e.g., aversive breathing load, affective touch, hunger, or pain) or by using top-down interoceptive attention instructions (e.g., “focus on your bladder”). No studies incorporated interoceptive accuracy, discrimination, or any other interoceptive behavioral measure into the neuroimaging analyses; future studies will need to establish how the neural representation of specific interoceptive dimensions differs in psychiatric disorders.
Additionally, in our meta-analysis, clinical sampling was limited to psychiatric disorders and related symptoms. The statistical constraints of our meta-analysis approach, which required studies on group differences in order to construct a map of convergent different activation between groups (
41,
45), required us to exclude the large number of studies on interoception in healthy individuals alone, which has been formative in the field’s mechanistic understanding of interoceptive processes (reviewed in reference
42). We also did not include, for example, pain disorders (
70,
71), functional gastrointestinal disorders (
72), functional neurological disorders (
16), or connective tissue conditions (
73), although, unsurprisingly, interoception also differs across these and other conditions involving a markedly different bodily experience. As such, our analysis cannot adjudicate whether differential activation in the mid-insula represents a common locus for all disorders where interoceptive differences are implicated or whether it is specific to the psychiatric conditions we examined here. That said, in chronic pain disorders, pain-related mental health indices predict quality of life over and above physical pain variables (
74,
75), suggesting (
20) that the role of interoceptive disruption in disorders not classically considered psychiatric may not be as distinct from the present data as one might assume. Nevertheless, the degree to which this role is distinct or overlaps with the role of disrupted interoception in psychiatric disorders remains to be determined.
Lastly, but crucially, many studies included in this meta-analysis, as in the wider neuroscience field (
76,
77), were underpowered to detect all but relatively large effect sizes. This increases the likelihood that at least some of the coordinates from studies included in our meta-analysis were false positives. However, the cluster-level thresholding we employed helps to mitigate the potential contribution of false positives: a simulation of 120,000 ALE meta-analyses shows that this technique can control for excessive contribution of single experiments as long as 17 or more experiments are included (
47). However, small sample sizes in contributing studies does mean that true effects could be excluded or underrepresented in our meta-analysis as a result of lack of power in the original studies (i.e., increasing the likelihood of false negatives in our study). This can only be remedied by future large-scale neuroimaging studies characterizing interoception in patient groups.