REL-1017 (Esmethadone) as Adjunctive Treatment in Patients With Major Depressive Disorder: A Phase 2a Randomized Double-Blind Trial
Abstract
METHODS
Study Design
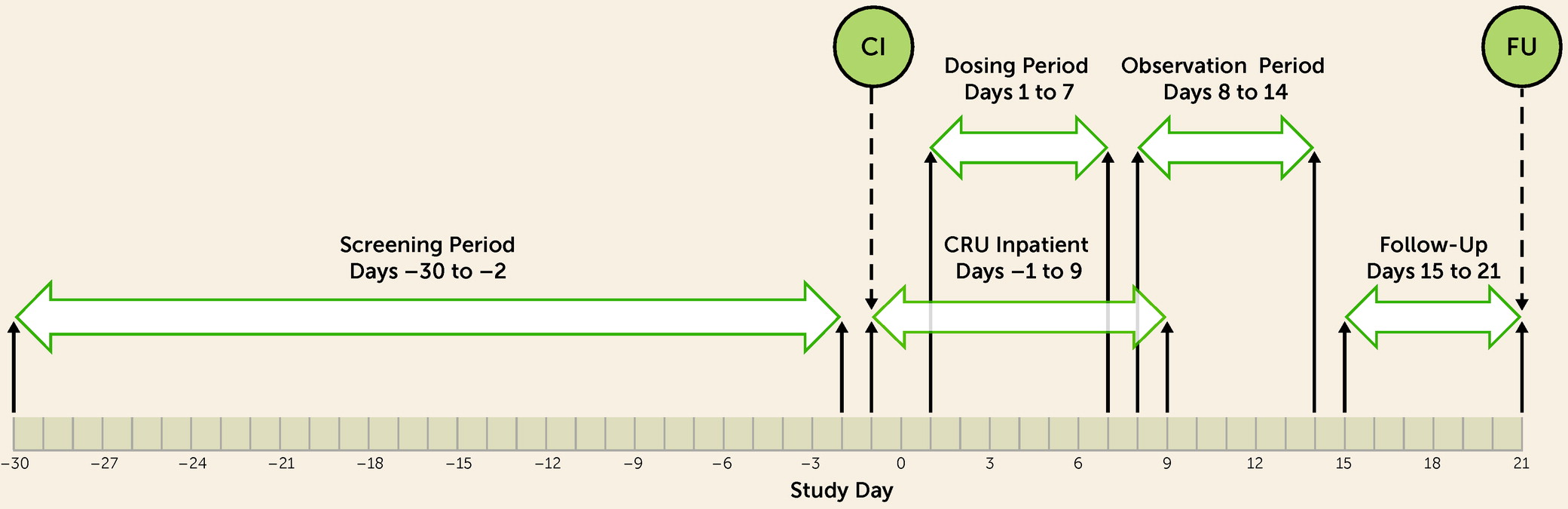
Participants
Randomization and Masking
Procedures
Outcomes
Statistical Analysis
RESULTS
| Characteristic | Placebo (N=22) | REL-1017 25 mg (N=19) | REL-1017 50 mg (N=21) | All Patients (N=62) | ||||
|---|---|---|---|---|---|---|---|---|
| Mean | SD | Mean | SD | Mean | SD | Mean | SD | |
| Age (years)b | 49.7 | 11.1 | 49.4 | 12.4 | 48.6 | 10.9 | 49.2 | 11.3 |
| N | % | N | % | N | % | N | % | |
| Sex | ||||||||
| Male | 11 | 50.0 | 11 | 57.9 | 12 | 57.1 | 34 | 54.8 |
| Female | 11 | 50.0 | 8 | 42.1 | 9 | 42.9 | 28 | 45.2 |
| Ethnicity | ||||||||
| Hispanic or Latino | 1 | 4.5 | 1 | 5.3 | 0 | 0.0 | 2 | 3.2 |
| Not Hispanic or Latino | 21 | 95.5 | 18 | 94.7 | 21 | 100.0 | 60 | 96.8 |
| Race | ||||||||
| Asian | 0 | 0 | 0 | 0 | 1 | 4.8 | 1 | 1.6 |
| Black or African American | 13 | 59.1 | 13 | 68.4 | 13 | 61.9 | 39 | 62.9 |
| Caucasian | 9 | 40.9 | 6 | 31.6 | 7 | 33.3 | 22 | 35.5 |
| Mean | SD | Mean | SD | Mean | SD | Mean | SD | |
| Body mass indexc | 29.02 | 4.27 | 27.66 | 3.33 | 27.66 | 5.00 | 28.15 | 4.26 |
| Baseline HAM-D score | 25.6 | 3.5 | 25.1 | 3.5 | 25.0 | 3.8 | 25.3 | 3.6 |
| Placebo (N=23) | REL-1017 25 mg (N=19) | REL-1017 50 mg (N=21) | All Patients (N=62) | |||||
|---|---|---|---|---|---|---|---|---|
| Variable | N | % | N | % | N | % | N | % |
| Patients with a serious adverse event | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 |
| Patients with a severe treatment-emergent adverse event | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 |
| Patients with at least one adverse event | 12 | 54.5 | 9 | 47.4 | 15 | 71.4 | 36 | 58.1 |
| Treatment-emergent adverse events occurring in three or more patients | ||||||||
| Constipation | 3 | 13.6 | 1 | 5.3 | 3 | 14.3 | 7 | 11.3 |
| Nausea | 2 | 9.1 | 1 | 5.3 | 2 | 9.5 | 5 | 8.1 |
| Diarrhea | 3 | 13.6 | 0 | 0.0 | 0 | 0.0 | 3 | 4.8 |
| Headache | 3 | 13.6 | 2 | 10.5 | 3 | 14.3 | 8 | 12.9 |
| Somnolence | 2 | 9.1 | 1 | 5.3 | 1 | 4.8 | 4 | 6.5 |
| Dizziness | 1 | 4.5 | 1 | 5.3 | 1 | 4.8 | 3 | 4.8 |
| Back pain | 0 | 0.0 | 1 | 5.3 | 2 | 9.5 | 3 | 4.8 |
| Measure, Time Point, and Group | N | Least Square Meanb | SE | Difference of Least Square Mean Drug Versus Placeboc | 90% CI | Effect Sized | pe |
|---|---|---|---|---|---|---|---|
| MADRS | |||||||
| Day 7 | |||||||
| Placebo | 21 | –8.7 | 2.3 | ||||
| REL-1017 25 mg | 19 | –17.4 | 2.5 | –8.7 | –14.3, –3.1 | 0.8 | 0.0122 |
| REL-1017 50 mg | 21 | –15.9 | 2.4 | –7.2 | –12.7, –1.8 | 0.7 | 0.0308 |
| Day 14 | |||||||
| Placebo | 20 | –7.4 | 2.4 | ||||
| REL-1017 25 mg | 16 | –16.8 | 2.7 | –9.4 | –15.4, –3.5 | 0.9 | 0.0103 |
| REL-1017 50 mg | 18 | –17.8 | 2.6 | –10.4 | –16.1, –4.6 | 1.0 | 0.0039 |
| SDQ | |||||||
| Day 7 | |||||||
| Placebo | 21 | –37.9 | 6.4 | ||||
| REL-1017 25 mg | 19 | –52.4 | 7.1 | –14.5 | –30.0, 1.0 | 0.5 | 0.1237 |
| REL-1017 50 mg | 21 | –52.9 | 6.6 | –15.0 | –30.1, 0.1 | 0.5 | 0.1017 |
| Day 14 | |||||||
| Placebo | 20 | –31.8 | 5.6 | ||||
| REL-1017 25 mg | 16 | –55.0 | 6.4 | –23.2 | –36.9, –9.4 | 0.9 | 0.0066 |
| REL-1017 50 mg | 18 | –58.6 | 6.0 | –26.8 | –40.1, –13.5 | 1.1 | 0.0014 |
| CGI-S | |||||||
| Day 7 | |||||||
| Placebo | 21 | –0.8 | 0.3 | ||||
| REL-1017 25 mg | 19 | –1.7 | 0.3 | –1.0 | –1.6, –0.3 | 0.7 | 0.0245 |
| REL-1017 50 mg | 21 | –1.7 | 0.3 | –0.9 | –1.6, –0.2 | 0.7 | 0.0253 |
| Day 14 | |||||||
| Placebo | 20 | –0.7 | 0.3 | ||||
| REL-1017 25 mg | 16 | –1.6 | 0.3 | –0.9 | –1.7, –0.2 | 0.7 | 0.0454 |
| REL-1017 50 mg | 18 | –2.0 | 0.3 | –1.3 | –2.0, –0.6 | 0.9 | 0.0043 |
| CGI-I | |||||||
| Day 7 | |||||||
| Placebo | 21 | 3.2 | 0.2 | ||||
| REL-1017 25 mg | 19 | 2.4 | 0.2 | –0.8 | –1.3, –0.2 | 0.8 | 0.0177 |
| REL-1017 50 mg | 21 | 2.3 | 0.2 | –0.9 | –1.4, –0.3 | 0.9 | 0.0072 |
| Day 14 | |||||||
| Placebo | 20 | 3.3 | 0.3 | ||||
| REL-1017 25 mg | 16 | 2.6 | 0.3 | –0.7 | –1.3, 0.0 | 0.6 | 0.0895 |
| REL-1017 50 mg | 18 | 2.3 | 0.3 | –1.0 | –1.6, –0.4 | 0.8 | 0.0109 |
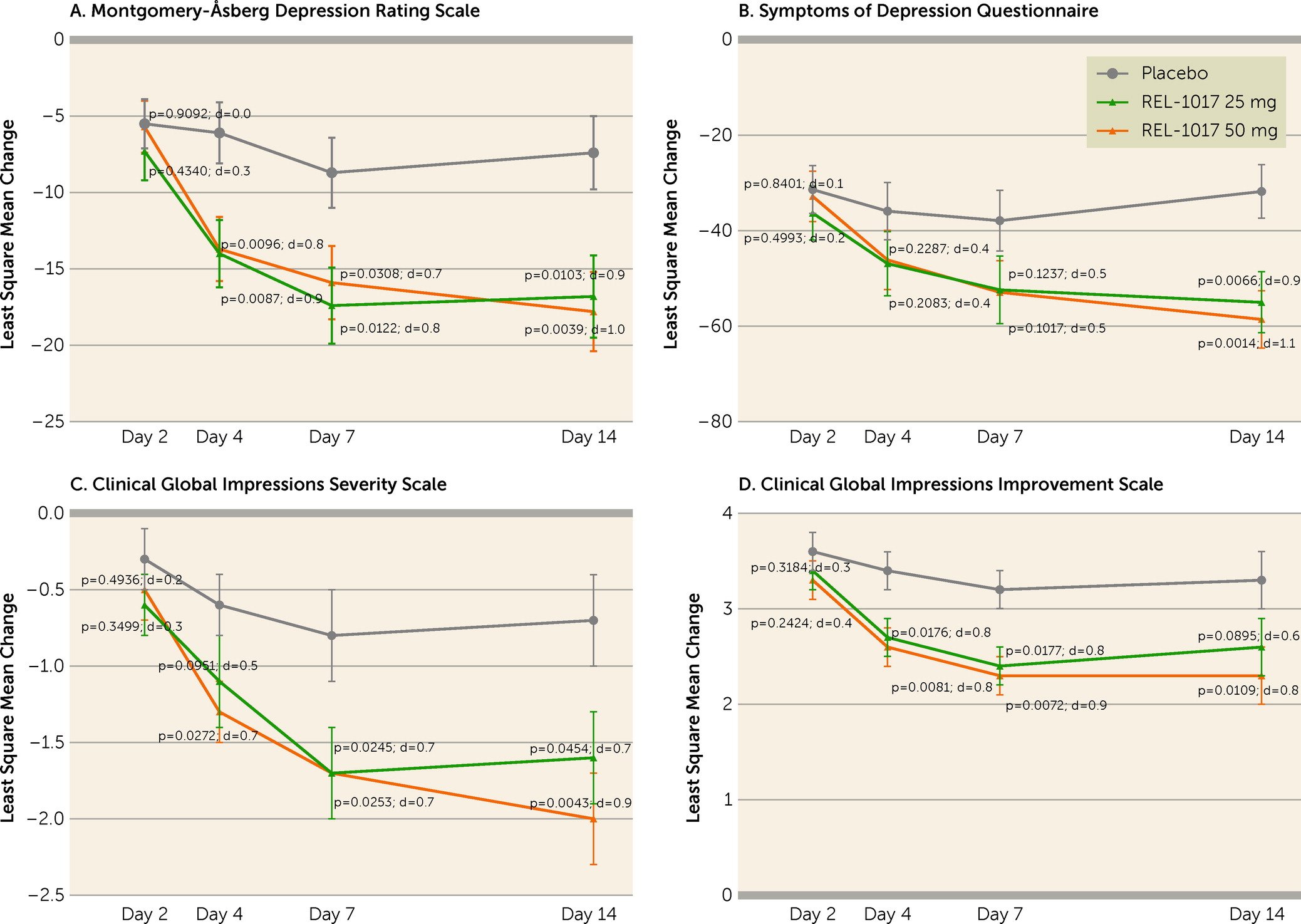
DISCUSSION
Footnotes
Supplementary Material
- View/Download
- 15.52 KB
- View/Download
- 691.69 KB
References
Information & Authors
Information
Published In
History
Keywords
Authors
Competing Interests
Funding Information
Metrics & Citations
Metrics
Citations
Export Citations
If you have the appropriate software installed, you can download article citation data to the citation manager of your choice. Simply select your manager software from the list below and click Download.
For more information or tips please see 'Downloading to a citation manager' in the Help menu.
View Options
View options
PDF/EPUB
View PDF/EPUBLogin options
Already a subscriber? Access your subscription through your login credentials or your institution for full access to this article.
Personal login Institutional Login Open Athens loginNot a subscriber?
PsychiatryOnline subscription options offer access to the DSM-5-TR® library, books, journals, CME, and patient resources. This all-in-one virtual library provides psychiatrists and mental health professionals with key resources for diagnosis, treatment, research, and professional development.
Need more help? PsychiatryOnline Customer Service may be reached by emailing [email protected] or by calling 800-368-5777 (in the U.S.) or 703-907-7322 (outside the U.S.).

