Depression is one of the most prevalent and costly mental health conditions (
1), with a public disease burden of staggering proportions (
2). While efficacious treatments have been available for decades, remission rates are low, relapse rates are high, and disorder prevalence rates remain notably consistent or increased, with only 12.7% of patients receiving minimally adequate treatment (
3). In recent years, two potential breakthroughs have ignited hopes for turning a corner in the clinical management of this disabling disorder. First, rapid-acting pharmacological agents—notably, intravenous ketamine (
4–
6)—have shown promise in dramatically reducing symptoms within the space of 2–24 hours, overcoming the sluggish response to conventional therapies (e.g., 4- to 6-week delays), which prolongs suffering, contributes to nonadherence, and fails to address urgent clinical needs (e.g., suicidality and other psychiatric crises). Second, intervention development has increasingly sought to take advantage of technology to increase patient access, reduce cost, and minimize aversive consequences through the use of automated, computer-based procedures (
7), including mechanistic treatments designed to modulate affective processing patterns directly in order to reduce symptoms (
8). The present study aimed to synergistically combine the benefits of these two approaches.
The rapidity of ketamine’s antidepressant effects offers the promise of a breakthrough in the way depression is managed, but this promise remains unfulfilled to date. Quickly dissipating effects after a single infusion in isolation (
9), coupled with substantial concerns regarding the safety and feasibility of repeated infusions (
10,
11), limit ketamine’s clinical impact. While substantial advancements have recently been made in understanding ketamine’s molecular mechanisms of action (
12,
13) and developing strategies to extend these effects pharmacologically (
14,
15), translation to safe, effective, novel pharmacology can be slow and uncertain. Furthermore, long-term pharmacological intervention is unlikely to be beneficial, feasible, and appealing to every patient (
16). A large majority of patients (e.g., up to 91% of depressed patients enrolling in drug trials [
17]) consistently express a preference for behavioral or combined behavioral/pharmacological treatments (
18,
19), in part reflecting the belief that such treatments will introduce new learning that will solve the core problem and instantiate lasting change (
19). Antidepressant pharmacological regimens are challenging to maintain in the long term because of patient discontinuation (
16) and the rarity of follow-up opportunities in community practice (
20).
Ketamine is posited to reverse the molecular signature of depression via synaptogenic and neuroplasticity effects at the molecular level (
12,
21,
22). Based on previous work, we have hypothesized (
23,
24) that these molecular effects may produce a corresponding neurocognitive shift, in which we can extend rapid mood relief during a window of opportunity using behavioral learning–based approaches—for example, by reinforcing adaptive patterns of cognition through automated training. Our study was designed to initiate training during a key clinical window of opportunity—1–4 days following a ketamine infusion, when euthymic mood and enhanced plasticity were expected—in order to consolidate beneficial processing patterns and prolong ketamine’s rapid mood effects.
In the context of depression, clinical effects of automated training paradigms, though showing some potential (
8,
25), are fairly unreliable, particularly in acutely depressed patients (
26,
27)—a situation that is likely exacerbated in the context of treatment-resistant forms of depression. We thus hypothesized that only after first “priming” brain plasticity with ketamine, the introduction and facilitation of new learning via automated methods might provide an efficient path to enduring relief for patients with treatment-resistant depression. Consistent with rising momentum in the creation of synergistic biobehavioral approaches to psychiatric management (including both ketamine [
28–
30] and other “psychoplastogenic” drugs [
31]), the present study was designed to push the boundaries of how rapid-acting antidepressant drug agents could be used clinically, that is, as short-term cognitive flexibility enhancers to promote learning that is antithetical to the depressed state. Ketamine infusion was used as the first step in a novel, efficient treatment algorithm designed to foster relief that would be both rapid and durable, simultaneously taking advantage of technology’s potential for low-cost, portable, safe, dissemination-ready interventions. This approach is in line with ongoing, similar efforts to pharmacologically augment automated cognitive training interventions in diverse populations (e.g., schizophrenia, cognitive aging) (
32–
36), but it targets a unique, affectively valenced aspect of cognition, namely, implicit self-worth.
An automated training paradigm specifically targeting implicit self-associations (“automated self-association training”; ASAT) was developed, rooted in our previous work suggesting that optimal synergy might be achieved when targeting this particular form of information processing after treatment with ketamine. Rapid shifts in implicit self-associations following ketamine treatment have been replicated in two previous studies (
37,
38), suggesting that ketamine creates the requisite malleability in information processing within this domain. To capitalize on this plasticity and to shift self-associations in a positive direction, we thus adapted existing appetitive conditioning paradigms known broadly as “evaluative conditioning” (
39,
40). Previous neuroimaging work also implicated rapid shifts following ketamine treatment in prefrontal and striatal network activity and connectivity (e.g., increased activation in the caudate and increased striatal–medial prefrontal cortex connectivity during face processing [
41]), which are widely implicated as substrates of appetitive conditioning in humans and rodents (
42), further suggesting potential mechanistic synergy (at the neurocircuitry level) for our selected approach when initiated following ketamine treatment. Finally, “depressive self-schemas” (negative self-beliefs) are among the most long-standing and well-documented cognitive mechanisms mediating depression (
43), suggesting strong clinical relevance for this mechanistic target.
In this first-of-its-kind study, our active/active combination of ketamine and ASAT was compared, in a randomized, double-blind, parallel-arm design, to control conditions that included each intervention component in the absence of the other (i.e., saline plus active ASAT; ketamine plus sham ASAT). This enabled a direct test of hypothesized complementary actions between ketamine and ASAT, in an effort to generate antidepressant action that was both rapid and enduring.
Methods
Participants
A total of 154 patients (ages 18–60) were randomized. All patients met DSM-5 criteria for major depressive disorder (see the
online supplement), and all patients reported moderate to severe levels of depression (a score ≥25 on the Montgomery-Åsberg Depression Rating Scale [MADRS] [
44]), lower-than-normative self-reported self-esteem (i.e., scoring outside of one standard deviation from the normative mean on the Cognitive Triad Inventory [
45] “self” subscale [
46,
47] and/or the Rosenberg Self-Esteem Scale [
48]), and at least one unsuccessful adequate trial of an antidepressant medication approved by the U.S. Food and Drug Administration (FDA) in the current depressive episode (assessed with the Antidepressant Treatment Response Questionnaire [ATRQ] [
49]). Any existing depression treatment regimens were required to be stably maintained beginning ≥4 weeks before screening (which equated to roughly 6 weeks before infusion date) and throughout the 30-day trial. See the
online supplement for further rationale and details of all inclusion and exclusion criteria, screening assessments, and study ethical oversight. See
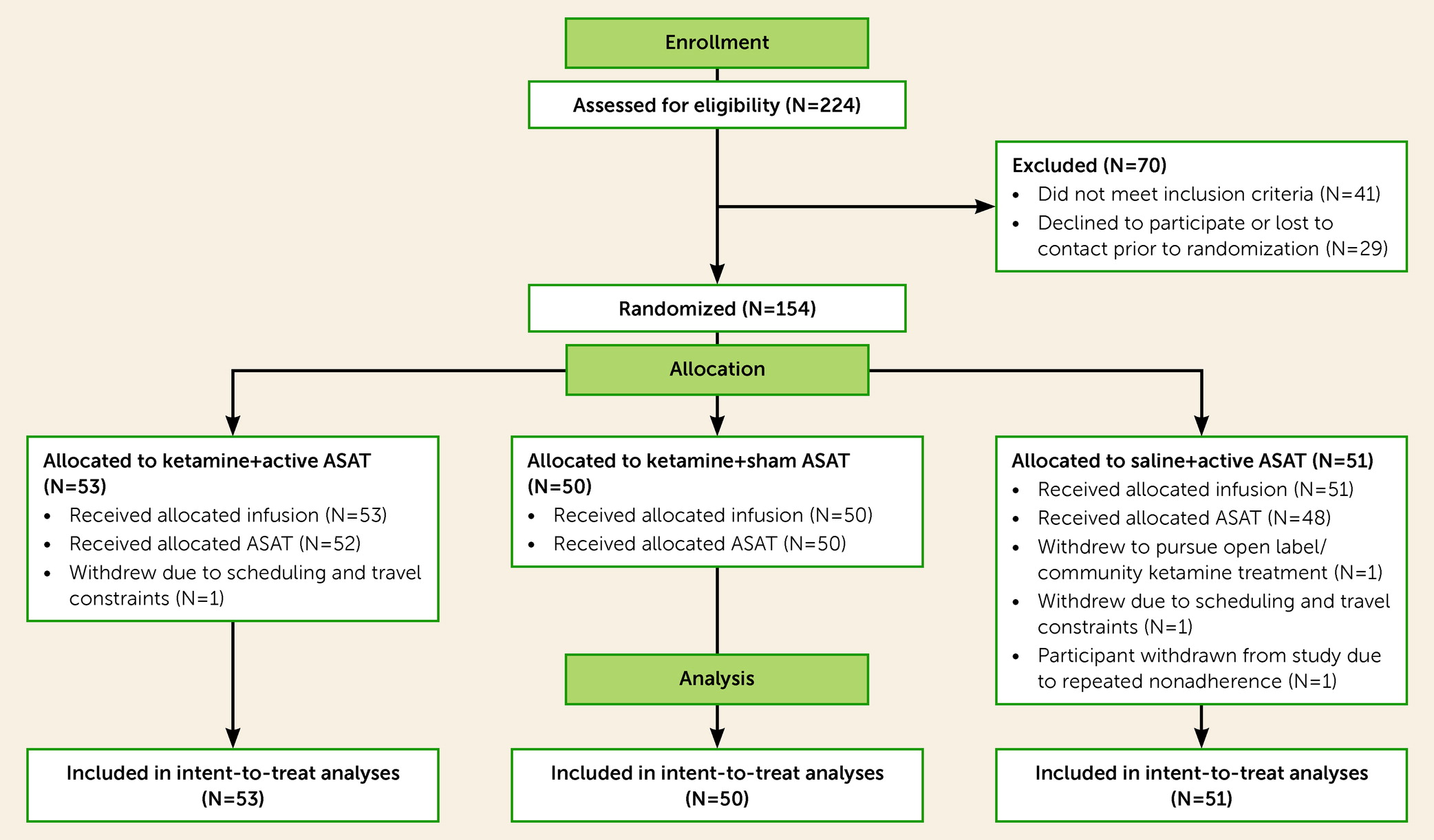
Figure 1 for the CONSORT flow diagram, which reflects high adherence and retention, and
Table 1 for descriptive patient characteristics.
To achieve the initial recruitment target of a sample of 150 patients, the study enrolled and randomized 154 patients, continuing enrollment until a final sample was acquired that included the full target of 150 patients who completed, at a minimum, an infusion day, a 24-hour assessment visit, and ≥75% of intended training (active or sham ASAT) visits, and were thus considered to have received their ASAT allocation (and labeled accordingly in the CONSORT flow diagram). All but two participants who surpassed this 75% threshold for their ASAT allocation received the full, eight-session dose of intended ASAT visits, per protocol (N=148 for full, per-protocol completers who received all allocated ASAT sessions). Thus, 96% of randomized participants were fully adherent with all intervention sessions, and 97% of all intended ASAT sessions were delivered.
A three-arm study design was selected to allocate all available resources toward the active/active (ketamine+ASAT) treatment arm relative to two crucial comparator groups, comprising each intervention component in the absence of the other—a conservative test of the active/active biobehavioral intervention’s combined impact. An inactive/inactive (no-treatment) arm was forgone in order to maximize statistical power for these more conservative and critical comparisons. An a priori power analysis was based on a principal interest in clinically meaningful, moderate (or larger) effects. The target sample sizes were selected to yield 80% power to detect moderate effects based on infusion phase comparisons (see “Statistical Analysis” below) of 100 ketamine and 50 saline patients (d≥0.49 and r≥0.31 using an alpha of 0.05), and based on comparisons of 50 patients in each of the ketamine+ASAT, ketamine+sham, and saline+ASAT conditions during the ASAT phase, where effects of d≥0.57 would be detectable with 80% power (using an alpha of 0.05). Power for primary mixed-effect analyses comparing treatment groups, which increases with additional repeated measures, was anticipated to be higher than for these simplified, single-point contrasts, making these power calculations conservative.
Assessment Measures and Timeline
In this primary clinical report from the trial, the focus is on the combined intervention’s impact on MADRS score, the study’s prespecified primary clinical endpoint for the acute (30-day) study period, to assess overall depression severity across the 30-day period when all other psychiatric treatments were held constant. A single, experienced master’s-level clinical rater administered the MADRS. The scale was administered at screening (used for eligibility determinations only), preinfusion baseline (infusion −1 hour; the clinical rater then left and was not present during any intervention procedure), infusion +24 hours (prior to the first session of active/sham ASAT), and at 5, 12, 21, and 30 days. A 1-year exploratory naturalistic follow-up, utilizing remote assessments acquired strictly through a distinct self-report symptom scale (the Quick Inventory of Depressive Symptomatology–Self-Report [
50]), remains under way, and data are not included in the present report. Neurocognitive/neuroimaging assessments of ketamine’s impact are undergoing analysis and will be included in forthcoming publications, along with secondary outcomes; however, one such cognitive measure (a “target engagement” measure of implicit self-associations) is analyzed in the
online supplement. At the final in-person visit (at 30 days), the adequacy of both patient and rater blinding, for both infusion and ASAT allocation, was assessed; see the
online supplement.
Interventions
A randomization table using permuted blocks of three or six, stratified by 1) biological sex assigned at birth and 2) modest versus severe treatment resistance (severe was defined as having had at least three unsuccessful FDA-approved antidepressant trials in the episode, per the ATRQ), was generated using SAS (SAS Institute; Cary, N.C.) by an independent statistical consultant at study start and was maintained by the study pharmacist, who, at the point of randomization, conveyed the ASAT allocation (but not the infusion allocation) to a single study staff member assigned to that patient. This single staff member was tasked with opening the correct computer program to deliver the ASAT sessions to that patient, but was not involved in, or present for, any outcome ratings. Blinding of all other study personnel was maintained until after the final patient in the trial had completed their day 30 assessment.
Participants were randomized in a 2:1 ratio to receive ketamine (0.5 mg/kg) or saline (50 mL 0.9% sodium chloride), administered over a 40-minute infusion, as in previous studies (e.g.,
51). All infusions were administered by blinded, licensed nurses in a medical hospital setting with immediate, emergency access to an Advanced Cardiovascular Life Support–certified team, oversight by a blinded study physician (R.H.H.), and safety and adverse event monitoring sustained for 4 hours after infusion. Adverse events (see Table S2 in the
online supplement) were mild to moderate, time limited, and consistent with the safety profile described in numerous previous trials using identical infusion procedures.
Automated Self-Association Training (ASAT)
Drawing on previous work utilizing “evaluative conditioning” to influence participants’ preferences and “liking” (
39,
40), active ASAT was designed to leverage Pavlovian conditioning in order to promote positive implicit self-associations and self-worth. Eight 15- to 20-minute sessions (delivered in a private research office setting, twice daily for 4 consecutive days, spaced by an intersession interval ≥20 minutes) were initiated 1 day postinfusion (after the 24-hour postinfusion assessment). In each of the eight sessions, both verbal and pictorial stimulus pairings were presented, both supraliminally (≥250 ms) and subliminally (12 ms), to practice and reinforce implicit associations between positive traits (e.g., “sweet,” “attractive,” photos of smiling actors; the unconditioned stimuli [US]) and self-referential stimuli (e.g., “I,” participant headshots; the conditioned stimuli [CS]). Incidental tasks such as a “lexical decision” task (indicating whether targets were real words or random letter strings) and a rapid mouse-tracking task (clicking as fast as possible on the position of stimuli) were used to enhance engagement and to promote semantic and visual processing of stimuli. Similar forms of evaluative conditioning have been found to alter implicit self-esteem (
40,
52,
53), mood reactivity to stress (
52), and pathological behaviors (anxious avoidance [
54], self-injury [
25]).
Sham ASAT consisted of the exact same computer tasks, but with predominantly neutral rather than positive US and non-self-relevant CS (words related to “others,” pictures of gender-matched strangers), designed to eliminate the possibility of unintended self-referential or iatrogenic negative learning, while providing a credible “brain-training” paradigm that controlled for all nonspecific factors (e.g., exposure to lexical and pictorial stimuli with a range of affective valences; incidental task demands; and time spent in the research setting) and was effective in facilitating adequate patient blinding (see Table S1 in the
online supplement). See the
online supplement for further technical details on the active and sham ASAT procedures.
Statistical Analysis
Intent-to-treat linear mixed-effects regression models were applied with continuous MADRS scores as the outcome, days since infusion as a random, continuous within-subject effect, and treatment allocation as a fixed, between-subject effect. Separate models were designed to test two discrete hypothesized linear patterns, based on 1) the infusion phase of intervention (two-way split: ketamine vs. saline), using preinfusion and 24-hour assessments only (assessments collected prior to onset of active/sham ASAT); and 2) the ASAT phase of intervention (three-way split: saline+ASAT vs. ketamine+sham vs. ketamine+ASAT), using assessment points from 24 hours (as a pre-ASAT baseline for these trajectories) through day 30.
In the infusion phase, we hypothesized rapid symptom decreases in the ketamine arm (relative to saline). For descriptive and clinical characterization purposes only, to facilitate comparisons with previous studies, dichotomous outcomes were defined for 24-hour responders (≥50% decrease from preinfusion MADRS baseline score) and remitters (MADRS score ≤9 [
55]). In the ASAT phase, the saline+ASAT arm was the reference group for hypothesis tests, and models included preinfusion MADRS scores as a covariate. We hypothesized a stable (main) effect extending from 24 hours to day 30 in the ketamine+ASAT arm (relative to saline+ASAT), with no group-by-time interaction. By contrast, for the ketamine+sham group, we hypothesized a linearly increasing trajectory of symptoms from 24 hours to day 30 (i.e., a group-by-time interaction relative to the reference group), consistent with the dissipation of symptom improvements within 1–2 weeks following a single infusion of ketamine (in isolation) that has been reported in previous ketamine trials (
4–
6).
All models included a random intercept and slope for participant to model patient-level trajectories over time and automatically account for missing data, which were minimal (5.1% of all intended observations). For interpretability, continuous variables were standardized. We report standardized beta coefficients (β) as a measure of effect size reflecting the number of standard deviations in the dependent measure that correspond to a one-unit change in the independent measure (e.g., group contrast), akin to Cohen’s d, with 95% profile likelihood confidence intervals. Analyses were performed using R, version 3.6.
Sensitivity analyses (see the
online supplement) probed for the robustness of findings when including the following covariates selected a priori: sex, age, treatment resistance (moderate vs. severe), and use of concomitant psychotropic medications (dichotomized as yes/no).
Results
Depression Severity
Infusion phase.
Consistent with previous reports, ketamine rapidly reduced MADRS total depression scores at 24 hours postinfusion (group-by-time interaction: standardized beta [β]=−1.30, 95% CI=−1.89, −0.70; t=−4.29, df=150, p<0.0001) (
Figure 2). This moderate to large linear effect corresponded to a 52% response rate and a 28% remission rate for the ketamine-treated participants, compared with a 25% response and 4% remission rate for the saline-treated participants (the number needed to treat for response was 3.7, and for remission, 4.2).
ASAT phase.
In intent-to-treat linear mixed models, depression scores in the ketamine+ASAT group remained stably low over the 30-day acute phase relative to those in the saline+ASAT group (β=−0.61, 95% CI=−0.95, −0.28; t=−3.62, df=148, p=0.0004) (
Figure 2), with no corresponding group-by-time interaction (β=0.009, 95% CI=−0.004, 0.021; t=1.39, df=568, p=0.164), suggesting durability of effect over the 30-day window. By contrast, depression scores in the ketamine+sham group followed an increasing linear trajectory from 24 hours to 30 days, as the postketamine effect gradually waned and depression score levels approached those of the saline+ASAT group (group-by-time interaction relative to the saline+ASAT group: β=0.015, 95% CI=0.003, 0.03; t=2.35, df=568, p=0.019). While the above intent-to-treat statistical tests of linear trajectories over the full 30-day period were used for statistical hypothesis tests, pairwise post hoc contrasts at day 30 were used to generate effect size point estimates for further description of clinical impact at this specific time point. These effect size point estimates suggested a small effect favoring ketamine+ASAT relative to both saline+ASAT (β=−0.38, 95% CI=−0.78, 0.027) and ketamine+sham (β=−0.31, 95% CI=−0.75, 0.13).
All the findings reported above were robustly upheld in sensitivity analyses controlling for covariates (see the
online supplement). No significant interaction (moderating) effects were observed between covariates and treatment allocation for either study phase (see the
online supplement).
Discussion
In this study, we found that automated self-association training—a novel, low-cost, fully automated, noninvasive, brief (eight sessions, ≤20 minutes per session), computer-based intervention—extended the rapid antidepressant effect of a single ketamine infusion for at least 30 days. In one of the largest ketamine randomized controlled trial samples to date, we replicated the well-established finding that intravenous ketamine exerts a rapid antidepressant effect in patients with treatment-resistant depression (
4–
6). Ketamine infusion appeared also to open a clinical window of opportunity, enabling the efficient uptake of positive self-representations (which were evident at the level of implicit cognition following ASAT; see the
online supplement) to protect against the return of depression over the subsequent month (
Figure 2). Symptoms in the saline+ASAT group, which received identical ASAT procedures in the absence of pretreatment with ketamine, were elevated relative to those in the ketamine+ASAT group throughout the 30-day follow-up period. Of note, symptoms following saline+ASAT also remained stably below pretreatment baseline, which could be attributable to a stand-alone impact of ASAT procedures (which were effective at altering implicit cognition, as noted above), or to an enduring, nonspecific impact of the overarching clinical research context (e.g., repeated depression assessments and staff interactions), or both. A second comparator group, treated with ketamine followed by sham ASAT, followed the expected trajectory when a single ketamine infusion has been given as a stand-alone treatment—rapid decrease followed by gradual return of depressive symptoms over the ensuing weeks (
4–
6). To our knowledge, this represents the first study to test a biobehavioral pairing of ketamine with a behavioral intervention that has included both a no-ketamine and a no-behavioral-intervention control condition. Likewise, clinical trials testing a wide range of posited synergistic treatments (e.g., behavioral treatments paired with “psychoplastogens” or other brain-based targeted approaches, e.g., neuromodulation) have routinely neglected one or the other of these two critical comparators (
24). Our design affords pivotal, novel evidence for the combined benefit of ketamine’s acute impact and the introduction of protective learning via ASAT, relative to patients’ pretreatment baseline symptoms and to either intervention component when given in isolation—providing a conservative test of efficacy. However, our design cannot provide estimates of the impact of the combined intervention (nor of each intervention component alone) relative to no intervention at all.
While we expect that a range of traditional and nontraditional behavioral treatments might work synergistically with rapid-acting pharmacotherapy, here we focused specifically on our novel ASAT approach, for both practical and scientific reasons. Practically, ASAT is fully automated and portable, and designed to be efficient, fitting well within the brevity (i.e., about 1 week) of ketamine’s posited window of opportunity. By contrast, a full course of either traditional or computer-based cognitive-behavioral therapy (CBT) typically requires 12 to 16 1-hour sessions delivered weekly, and meta-analyses suggest that optimal computer-based CBT requires supplemental therapist coaching (
56). Here, 30–40 minutes of ASAT for 4 consecutive days yielded effect sizes for ketamine+ASAT (relative to unimodal treatment conditions) that were moderate across the entire follow-up interval, but small in point estimates at the final assessment point (day 30). Further work will be needed to understand the patient perspective on the cost-benefit ratio of such gains vis-à-vis other, conventional treatment options (e.g., psychotherapy) and to assess adherence in clinical settings. However, we observed exceptional adherence (97%) with ASAT sessions in our research context, which is consistent with the high adherence and high ratings for acceptability and satisfaction observed previously for other fully automated therapies, across both research and clinical contexts (
7,
57–
59).
Scientifically, a robust evidence base suggests that implicit and explicit forms of cognition are distinct entities, and that implicit cognition (e.g., implicit self-concept) could be a more robust predictor of future behavior than explicit thought content (
60). ASAT is designed to efficiently and directly target implicit cognition, yielding an innovative approach with the potential to profoundly shape future behavior and experience. Furthermore, by targeting a unitary and well-defined implicit cognitive mechanism, ASAT limits the influence of heterogeneous and nonspecific factors that are likely influential in many traditional behavioral interventions, and for which the learning that occurs within a brief window may be more difficult to predict and constrain (e.g., positive vs. negative therapy session experiences). Broadly, evaluative conditioning techniques have a large and robust literature in healthy control subjects (
61), and in clinical samples, they have previously shown replicated acute clinical effects even in an at-home (smartphone) delivery modality (
25). Although in this initial test of ASAT we administered the training in a research lab environment, the ability to both achieve and maintain gains via portable, highly dissemination-ready techniques is a crucial criterion for overcoming intractable barriers to access to care, particularly for underserved communities (
62), representing a critical future direction for this work.
Given budgetary constraints, all available resources were allocated to determining the advantage of the active/active biobehavioral treatment combination over its component parts delivered in isolation, providing a conservative test of efficacy focused on establishing the necessity of each active component, but negating the ability to determine intervention efficacy in relation to no treatment. Functional unblinding was evident for the infusion condition (see Table S1 and further discussion in the
online supplement); clinical effects observed for ketamine versus saline may thus have been amplified by expectancy. Importantly, because adequate ASAT condition blinding was achieved (see Table S1 in the
online supplement), such effects are unlikely to have factored strongly into the subsequent extending effect of ASAT—the primary, novel focus of the present work. While ASAT achieved its hypothesized effect of extending the initial rapid improvements induced by ketamine, it did not produce incremental improvements over and above ketamine’s rapid effect, and response and remission rates leave substantial room for further improvement of efficacy. Our design cannot directly inform key clinically relevant questions regarding dose-response effects, treatment schedule optimization, and the trade-off between treatment efficiency and obtaining (and sustaining) maximal benefit. We erred on the side of front-loading and maximizing ASAT administration during the acute postketamine window of opportunity, within the perceived confines of feasibility and patient burden, while preserving spaced practice (i.e., 20-minute intersession breaks) in an effort to enhance learning (
63). While this initial effort focused on quantifying 30-day symptom trajectories following this highly efficient (5-day) intervention package, future work involving repeated ketamine doses, higher overall doses of ASAT, and/or subsequent ASAT booster sessions would expand the clinical relevance of the present work. Our ongoing, exploratory, naturalistic 1-year follow-up could yield further insights regarding durability and time to relapse, but for ethical reasons we did not ask patients to refrain from freely making treatment changes and additions during follow-up. Future work could also assess the feasibility and impact of targeting additional cognitive-affective associations beyond self-worth (e.g., perceptions of others, the future, etc.), which might enhance effect sizes and/or enable a personalized, modular approach that could be applicable transdiagnostically.
Finally, although clinical trial eligibility criteria were designed to emulate real-world treatment-seeking depressed samples (e.g., stable medications maintained rather than discontinued, and few exclusions based on medical or psychiatric comorbidities), the results may not robustly generalize to patients with distinct characteristics, including those with greater or lesser degrees of treatment resistance, with bipolar depression, or with comorbid moderate to severe substance use disorders. Critically, we are also unable to elucidate what the intervention’s efficacy and feasibility might be in samples with greater racial and ethnic diversity than ours had.
Conclusions
The global burden of depression is extremely high and is expected to continue to increase within the current context of a significant pandemic. There is an urgent need for novel treatment approaches, particularly those that can provide relief efficiently and at scale. If the present results can be replicated, this novel, integrative treatment may provide a method to urgently bring relief and to efficiently extend this relief via safe, low-cost, portable techniques. Alongside our fully automated ASAT intervention, ketamine infusion may likewise be more dissemination ready than many alternatives, with a favorable safety profile for isolated infusions and wide international medical usage in both anesthetic and subanesthetic applications. Additional efforts are warranted to create, and then exploit, rapidly induced neurobiological states (such as enhanced neuroplasticity) as clinical windows of opportunity in which to introduce new, protective learning.
Acknowledgments
The authors are grateful to the study participants for their time and dedicated collaboration in this work. The authors also gratefully acknowledge Joseph Franklin, Ph.D., Chadi Abdallah, M.D., Ph.D., Satish Iyengar, Ph.D., and Lisa Parker, Ph.D., for their assistance with this work.