In April 2022, the Substance Abuse and Mental Health Services Administration (SAMHSA) announced five core priorities for the upcoming year, including overdose prevention and performance measurement. Performance measurement has lagged in the behavioral health field, especially for opioid use disorder (OUD) (
1), compared with other areas of medicine (
2). The Health Effectiveness and Data Information Set (HEDIS) quality measures for substance use disorders (
3) adopted by the Centers for Medicare and Medicaid Services (CMS) are the most widely used measure sets by payers (
4); measure selection has important implications for health networks, providers, documentation, and reimbursement. While widely used, the HEDIS measures for substance use disorders grew primarily out of a consensus model from the late 1990s rather than rigorous data analysis (
5,
6). Empirical studies are therefore greatly needed to guide measure development and clinical validation to improve patient outcomes at the system level and reduce overdose death.
There are two primary evidence-based interventions for reducing opioid-involved overdose deaths among patients with OUD: medication initiation and medication retention (
7). While patients with OUD are receiving medication for OUD (MOUD), most commonly buprenorphine (
8,
9), their risk of death declines by 66%–80% (
10,
11). MOUD is the gold standard for OUD treatment and are widely promoted by federal health agencies. These two stages, medication initiation and retention, also undergird the OUD Cascade of Care (
12).
However, rather than focusing specifically on evidence-based treatment with MOUD, many existing quality measures are intended to apply more generally to any substance use disorder, including those for which medication-based treatment is not available (
1). For example, the widely used HEDIS measure of Engagement of Alcohol and Other Drug Abuse or Dependence Treatment, endorsed by the National Quality Forum (NQF #0004) (
13), applies to a broad spectrum of substance use disorders and utilizes professional encounters as the measure of success, requiring two outpatient visits or other professional services within 34 days of an initial visit. There is a need to assess how well this general measure specifically applies to individuals with OUD initiating MOUD. For example, are two professional visits within 34 days from an initiation visit for MOUD a necessary condition to achieve minimally adequate MOUD retention?
A generally accepted measure of minimally adequate MOUD retention is incorporated in a measure of pharmacotherapy retention recently endorsed by the NQF (NQF #3175) (
14), which specifies that patients initiating a medication, such as buprenorphine, should be continuously retained for a minimum of 6 months. Ideally, clinicians and payers could identify at treatment outset which patients are at risk for dropout before reaching this minimum duration of care.
To investigate the predictive value of early treatment response on 6-month treatment retention, we applied the HEDIS engagement quality measure (NQF #0004) to patients in a multisite, multistate buprenorphine maintenance treatment program. We hypothesized that patients who successfully met the engagement measure would be significantly more likely to be retained through 6 months (NQF #3175). We additionally evaluated whether successful treatment engagement was associated with even longer durations of care (i.e., 12 months and 24 months) and hypothesized that the association would persist but be attenuated.
Methods
We analyzed data from a multisite buprenorphine clinic network (
15), primarily managed by advanced practice professionals and supervising physicians across eight states from January 1, 2011 through April 15, 2019, with patient diversity broadly representative of buprenorphine patients nationwide, predominantly male and non-Hispanic White (
16,
l7). The partner site implemented systemwide protocol-based best practices in addiction treatment, escalating the level of care (i.e., frequency of visits) as needed to help patients achieve clinical stability with care coordination between case managers, physicians, and advanced practice clinicians. Patients were stratified into risk categories based on treatment response (i.e., opioid-free urine drug screen) to determine the number of visits per month, with visits typically conducted weekly or twice weekly in the first month of care. We collected longitudinal clinical data from the unified electronic health record (EHR).
We included individuals initiating a new buprenorphine care episode between January 1, 2011 and March 31, 2017 at provider sites and followed all care episodes for up to 24 months. We limited care episodes to patients completing their intake visit who had not received care at any partner site in the preceding 90-day period in order to identify new care episodes for OUD. The exposure was a dichotomous variable of whether patients satisfied the HEDIS engagement quality measure. In this setting, engagement was defined as two additional in-person outpatient clinical visits within 34 days of the intake visit.
Our primary analysis investigated probability of treatment discontinuation during the 180-day period following admission based on engagement status. We defined treatment discontinuation as a gap of 60+ days in visits, consistent with clinic policies and prior literature (
16–
18). We attributed the last day in care to the final clinic visit date. We calculated both absolute percentage differences in likelihood of successful retention, as well as adjusted odds ratios using logistic regression based on engagement status that adjusted for age, sex, and other baseline patient characteristics including initial drug test results, hepatitis C status, and HIV status. We repeated these analyses for retention at 12 and 24 months as secondary outcomes.
This study followed the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) reporting guidelines (
19) and was approved and deemed exempt from requiring informed consent by the New York State Psychiatric Institute IRB.
Results
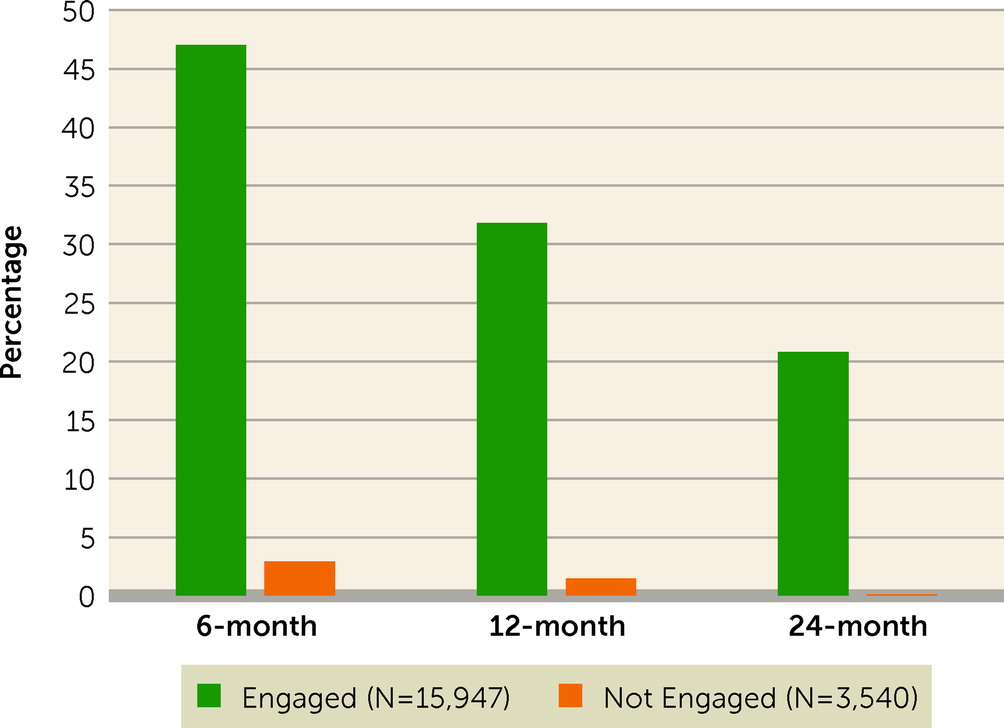
Our analysis identified 19,487 individuals meeting study eligibility criteria. The average age was 35.7 (SD=10.5 years), and 57.5% male. Among these new patients, 16,063 (82.4%) successfully engaged in care, and 3,424 (17.6%) did not. Among those successfully engaging in care, 47.0% remained in care for a minimum of 6 months versus 2.9% of those who did not meet measurement criteria for initial treatment engagement, yielding an unadjusted odds ratio of 29.1 (95% CI=23.9–35.6). This relationship between engagement and successful retention persisted but was attenuated for longer periods of retention at 12 months and 24 months (31.8% versus 1.5% and 20.8% v. 0.01%, respectively) (
Figure 1, data not shown in figure). In adjusted analyses, those who engaged compared with those who did not engage had 20.7 times (95% CI=16.8, 25.5) the odds of 6-month retention. This relationship persisted but was attenuated for longer periods of retention (
Table 1). Odds of 6-month retention were also increased for women (adjusted odds ratio=1.32, 95% CI=1.23–1.41), adults aged 50–64 years versus those under 30 years (adjusted odds ratio=2.02, 95% CI=1.80–2.26), those first testing positive for buprenorphine (adjusted odds ratio=1.88, 95% CI=1.75–2.01) compared with their respective reference groups (
Table 1). Patients testing positive for cocaine use were less likely than those testing negative to be retained at 6 months (adjusted odds ratio=0.51, 95% CI=0.47–0.57) as well as those positive for hepatitis C at treatment entry (adjusted odds ratio=0.82, 95% CI=0.76–0.89). HIV status was not significantly associated with retention.
Discussion
While we found that almost half of patients who successfully met HEDIS criteria for treatment engagement were subsequently retained in care for a minimum of 6 months, the more striking finding was that only a nominal 2.9% of those who did not engage remained in care at 6 months. This finding is clinically meaningful and could guide intervention development to prioritize stabilization of high risk patients early in treatment.
Results indicate treatment engagement is a threshold process that appears to be a generally necessary condition for adequate MOUD retention at 6 months. Monitoring HEDIS engagement among OUD populations initiating MOUD may help to identify and address barriers, facilitators, and disparities in clinical outcomes. Potential barriers include co-occurring but untreated psychiatric conditions and substance use disorders; logistics related to navigating work schedules, childcare arrangements, and travel; and underdosing of MOUD (
20). Interventions including integrated and coordinated care capable of simultaneously treating multiple conditions (
21) in a culturally competent manner (
22); care coordination services, peer navigators, and transportation and social service assistance (
23,
24); telehealth with remote visits; and typical buprenorphine dosing of a minimum 16 mg daily (
20,
25) may improve early engagement.
Value based contracts between providers and insurers that reimburse these interventions might enable scaling access to wraparound services. While generic SUD treatment disorder measures such as NQF #0004 appear to be relevant to the process of care for individuals with OUD, development and deployment of additional measures that are specific to the OUD population and to receipt of evidence-based treatments for this disorder, such as MOUD, is also needed to support data-driven continuous quality improvement. Given that patients testing positive for cocaine at intake were half as likely to retain in care, incorporating contingency management for stimulant use disorder among patients with OUD, payments for which are newly allowable by the US Department of Health, may better stabilize early clinical outcomes.
Our findings are subject to several limitations. While our patient population was similar to those in other multisite observational studies of buprenorphine patients (
16,
17), it was predominantly non-Hispanic white. Results may not generalize to other patient populations, and we were unable to account for patients who transferred care to other providers, although our prior research suggests this represents fewer than 20% of patients (
15). Additionally, while we did not account for buprenorphine dose, all clinics’ protocols guided for a typical daily maintenance dose of 16 mg. To be able to follow all patients for a minimum of 24 months, the study inclusion window closed in mid-2017, prior to the meteoric rise of fentanyl in the illicit drug market. Further, we were unable to account for censoring due to mortality.
Ongoing research is needed to identify patients at greatest risk for overdose, and to develop and refine performance measures that can help guide clinical care decisions, care networks, insurance plans, and administrative data reporting efforts. Rates of fatal opioid overdose have worsened in the last 3 years, particularly among vulnerable subpopulations such as Black individuals who may experience heightened barriers to treatment engagement and retention. We hope this study underscores the need for building an empiric evidence base for optimizing treatment pathways and related performance measures for the diverse array of patients with OUD to help reduce overdose risk.