The potential for misuse of benzodiazepines and benzodiazepine-related drugs (hereafter, BZRAs) has been a source of controversy for over five decades. These medications can be used for patients with anxiety, panic and sleep disorders, and seizure disorders (
1). They are commonly coprescribed with antidepressants and antipsychotic medications to treat the associated anxiety and sleep disturbances of these psychiatric disorders. When carefully prescribed to patients who are not substance abusers, benzodiazepines are rapidly effective and safe. The known pharmacology of these medications adds to their safety by allowing selection of a particular benzodiazepine based on elimination half-life and cytochrome enzyme metabolism. However, benzodiazepines are not without risks. They share dose-dependent side effects with other sedative-hypnotic drugs and may augment both positive and negative side effects of the primary medication. These include sedation, unsteadiness (falls in the elderly), mild physiological dependence with chronic use, and mild to moderate discontinuation symptoms when abruptly discontinued. Whether long-term benzodiazepine use interferes with memory and other cognitive abilities is controversial, but the available evidence suggests that benzodiazepines do not cause Alzheimer’s disease (
1).
However, weak science (
2), alarming FDA black box warnings, and media reporting have fueled an anti-benzodiazepine movement that at times even portrays appropriate BZRA prescribing as a gateway to long-term dose escalation, tolerance, and drug misuse. This has created an atmosphere of fear and stigma among patients, many of whom can benefit from such medications.
In this issue of the
Journal, Rosenqvist et al. (
3) report on the largest, longest, and most convincing country-wide study (N=950,767 BZRA recipients) of long-term use and BZRA dose escalation. Their Figure 2 provides striking evidence that is contrary to the widespread belief that medical use of BZRAs leads to long-term use and misuse: 85% of initial BZRA recipients discontinued the medications within 1 year, and 97% had stopped all BZRA use by 7 years. Additionally, dose escalation was very low. Among the 5% with continuous use over 3 years, less than 7% had dose escalations above recommended levels; this equals 0.35% escalating doses among almost 1 million patients starting BZRAs. This small magnitude is especially important to bear in mind when considering the results in the authors’ Table 2, since readers may be inclined to overinterpret the estimated fraction of patients who are escalating doses. These long-term recipients of BZRAs remain a tiny and select subgroup of all treated patients. Predictably, prior psychiatric morbidities and substance use disorders (including opioids) predicted dose escalation. The authors appropriately conclude that BZRAs are infrequently associated with long-term use and dose escalation.
These findings are largely consistent with decades of smaller studies in several countries: Our early study of BZRA dose escalation among 2,440 Medicaid recipients in New Jersey with at least 2 years of reported use that found that 1.6% of patients escalated to high doses (
4). A study of 81,945 BZRA recipients in Norway reported that only 0.9% ended up using >2 defined daily doses (
5). And a study of 12,598 patients in Canada recorded that 3.1% of patients who were prescribed benzodiazepines for at least 2 years escalated to doses higher than 40 diazepam milligram equivalents (
6).
Much of the previous research on outcomes of BZRAs (and other psychoactive medications) exaggerated drug harms on the basis of weak cross-sectional pharmacoepidemiologic designs that did not control for confounding by indication. This common bias occurs when physicians preferentially treat sicker or older patients. Conditions such as dementia, drug abuse, and other chronic illnesses often cause the adverse outcome, not the treatment itself. These biases are often hidden because sickness and frailty are poorly measured in common research and insurance databases.
There are many examples of confounding by indication in research on psychoactive medications. One notable example is the uncertain relationship between BZRAs and hip fractures. At first glance, this effect seems reasonable given BZRAs’ sedating effects in the elderly, possibly causing falls and fractures. The initial landmark case-control study of the effects of BZRAs on hip fracture rates that started the modern field of pharmacoepidemiology is a case in point (
7). That study compared BZRA recipients to nonrecipients without adequately controlling for the preexisting increased risk of fractures among BZRA recipients. Prior research had already shown that patients who start on BZRAs are at 29% increased risk of hypertension, 45% increased risk of joint problems (an obvious unmeasured confounder of fractures), a 50% increase in poor health, and a 36% excess risk of smoking as compared to patients in a similar age group who do not start on BZRAs. There are more such confounders in other studies (
8).
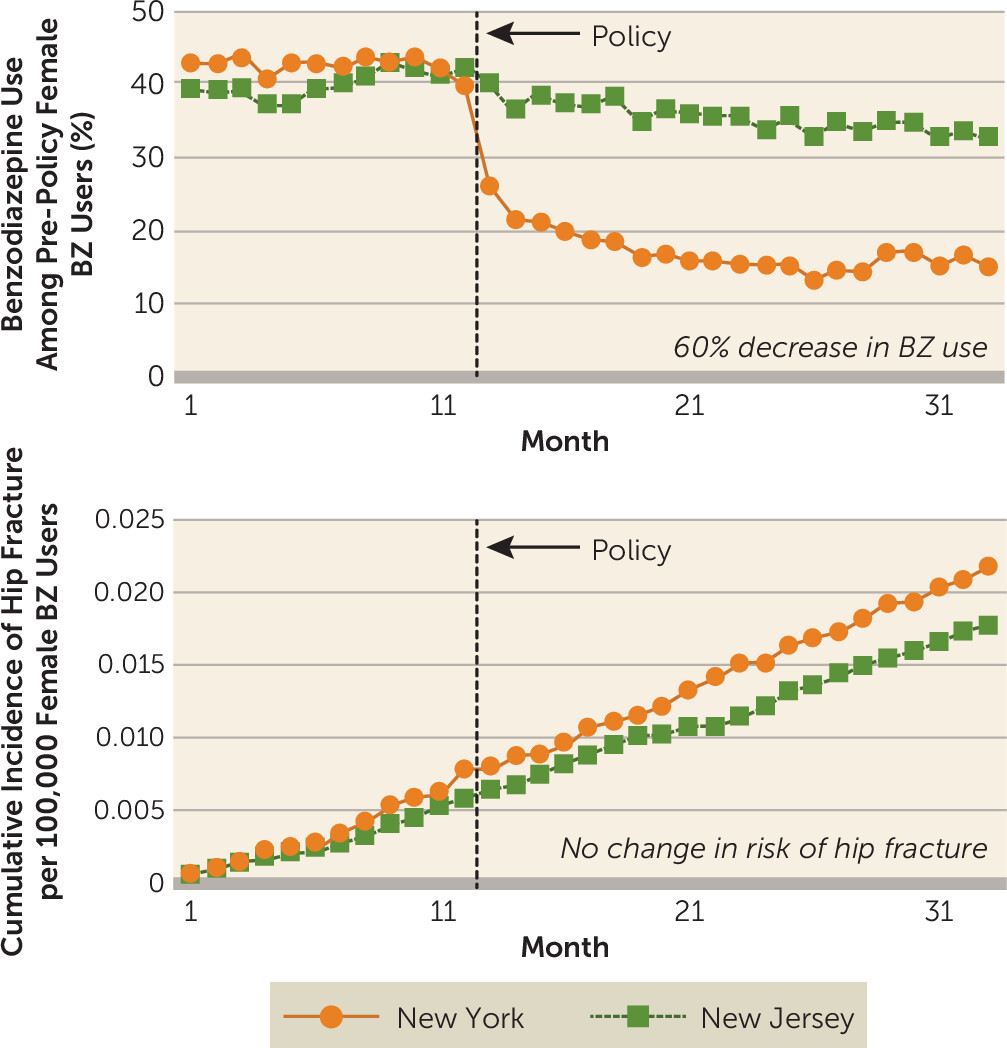
When researchers used a much more valid longitudinal design that is resistant to confounding by indication—the interrupted time series with controls—to measure the effects of a natural experiment in which a new state regulation restricted BZRA prescriptions, where the researchers examined outcomes among nearly 100,000 elderly Medicaid enrollees (the regulation was partially based on the above-mentioned biased case-control study), there were no measurable effects on rates of hip fractures in the population (
9). The findings from this study are clear: an almost 60% instantaneous and sustained decrease in BZRA use, and no reduction in risk of hip fracture (see
Figure 1 for rates among women). Of course, this finding does not negate the possibility of individual cases of BZRAs contributing to falls and fractures.
Even systematic reviews, intended to provide the totality of strong evidence on the intended and unintended effects of medical and health policy interventions, sometimes fail to control for bias due to confounding by indication. One such analysis of the effect of BZRAs on hip fractures (
10) failed to consider most of the common confounders cited above, so its findings of effects of BZRAs on rates of hip fracture are uninterpretable.
Exaggerated fears of BZRA misuse have generated an increase in worldwide regulations that have sharply reduced both essential and questionable BZRA use. One example is the New York triplicate prescription program regulation that in 1989 began requiring, for all BZRA prescriptions in the state, that a third copy of the prescription be forwarded to the New York State Department of Health. Fear of sanctions had chilling, unintended effects on quality of care. BZRA use immediately dropped by over 50%. Studies using robust designs show several notable effects. First, there were larger reductions in appropriate use than in problematic use (
11). Second, other covered and potentially more toxic drugs, such as meprobamate, were substituted for BZRAs, and abrupt discontinuation of BZRA treatment sometimes resulted in withdrawal symptoms or the return of symptoms of previously treated anxiety disorders (
12). Third, there was a sudden 50% reduction in BZRA use among some of the most vulnerable patients with major psychiatric disorders, such as schizophrenia, schizoaffective disorder, bipolar disorder, epilepsy, and panic disorder (
11) (it is noteworthy that psychiatric disorders are also associated with long-term use in the Rosenqvist et al. study). And fourth, racial disparities in treatment worsened (
13); patients living in Black neighborhoods had the lowest baseline rate of BZRA use but experienced the highest rates of discontinuation of nonproblematic use.
The large body of research on the unintended effects of the New York triplicate prescription program may have influenced its termination by the state, which now has replaced it with electronic surveillance. Unfortunately, some governments have implemented more draconian policies that completely stopped reimbursement of BZRAs in health insurance programs (e.g., Medicare Part D [
14] and the Netherlands [
15]), with obvious consequences for patients with seizure disorders, panic disorders, and serious mental illnesses for whom BZRAs may represent essential treatments. These policies had the intended effects of reducing BZRA use but also various unintended effects: increased rates of hip fractures among frail nursing home patients; substitution to more expensive, sedative drugs of questionable appropriateness, including powerful antipsychotic agents (
16); increased out-of-pocket costs; and possible dangerous withdrawal from chronic BZRA use among Medicare/Medicaid crossover patients who had BZRA coverage when the drugs were de-listed in the Medicare drug benefit (
14).
In summary, while BZRAs can pose risks, their mechanisms of action are known, and their appropriate use can provide substantial improvements in patient functioning and quality of life. The Rosenqvist et al. study, the largest and longest to date, should put an end to the unfounded assertions that appropriate BZRA prescribing often leads to dose escalation and drug misuse. Blunt policy instruments to reduce both appropriate and problematic care sometimes harm health outcomes in vulnerable patients. Policies must be based on better-designed studies that can control for bias. Finally, policy makers need to identify risks and benefits of strategies to reduce inappropriate psychoactive drug use before they are implemented.