Anxiety disorders are the most common of mental illnesses (
1), yet their pathophysiology remains poorly understood. Despite over two decades of neuroimaging research, which has outlined some of the key brain circuitry underpinning anxiety-relevant processes (
2,
3), we have yet to find robust brain-based biomarkers of anxiety. This has in part contributed to the cutbacks and discontinuation of drug discovery anxiety research seen in the private sector (
4).
One of the methodological factors contributing to a lack of robust markers derives from issues of statistical power. Some have argued that establishing robust brain-behavior relationships demands thousands of participants (
5). Anxiety research has not traditionally used designs of this magnitude. Smaller-scale studies are undoubtedly essential for scientific discovery (
6), but our neuroscientific models of anxiety have faced minimal validation against highly powered samples. Studies that attempt this have not always replicated key findings, such as an association between resting-state functional connectivity of the amygdala and self-reported trait anxiety (
7,
8), emphasizing the necessity of scrutinizing anxiety theory against larger data sets.
From a conceptual standpoint, a further hindrance to the identification of robust biomarkers may be from the very operationalization of anxiety as a unity construct, which has carved artificial boundaries with other forms of mental illness. The field has started to move toward a transdiagnostic approach which acknowledges that anxiety has shared mechanisms with other disorders, such as depression, as well as unique, disorder-specific features (
9). On the other hand, while researchers have started to broaden the lens to encapsulate cross-disorder mechanisms, far less work has focused on idiosyncratic features underlying subtypes of anxiety disorders. Meta-analytic evidence supports the notion of common circuitry shared across anxiety disorders (as well as adaptive anxiety and fear [
2,
3]), but we know far less about how, or indeed if, disorder subtypes differ. In sum, despite an increased uptake in transdiagnostic approaches, there is a clear need for exploring neuroimaging-based stratification of anxiety subtypes.
In this issue, Hilbert et al. (
10) report on the largest study of its kind to investigate brain structure differences in phobias, focusing on two subtypes: animal phobia and blood-injury-injection phobia. Leveraging multisite data from over 4,400 participants (2,991 healthy volunteers and 1,452 volunteers with a phobia), the researchers’ preregistered mega-analysis tested for phobia-associated differences in brain structure (i.e., subcortical volume, cortical surface area, and cortical thickness) relative to healthy control subjects. They found an overall reduction in subcortical volumes, namely, the caudate, putamen, and hippocampus for individuals with a phobia (vs. healthy controls), except for the pallidum, which was generally larger in the phobia group. These effects appeared primarily driven by participants with animal phobias (N=739). Conversely, participants with a phobia demonstrated predominantly increased gray matter thickness and surface area across various cortical regions, such as the medial orbitofrontal cortex, frontal pole, and occipital regions. Orbitofrontal thickening was particularly pronounced among participants with blood-injury-injection phobia (N=182). Many of these effects survived a battery of robustness tests, were of a magnitude higher than those seen in studies of generalized and social anxiety disorders, and corroborate the authors’ prior findings in smaller samples (
11). Combined with preregistered hypothesis testing and a relatively large sample size, Hilbert and colleagues’ findings give optimism to the idea that identifying robust brain-based measures of pathology is achievable in anxiety research.
Identifying biomarkers is, of course, only the start; to advance our theoretical understanding, we need to map markers onto underlying mechanisms (
12). Indeed, in stark contrast with the commonly held view of the amygdala as the “fear center” (
13), there were
no differences in amygdala volume between the healthy and phobia samples. This does not preclude the functional involvement of the amygdala, nor morphology of amygdala subnuclei, in anxiety-relevant processes, but it begs the question of how and when these neuroanatomical differences in phobia impact brain activation. To this end, a meta-analysis of activation to emotion tasks among phobia patients, published in the
Journal in 2021 (
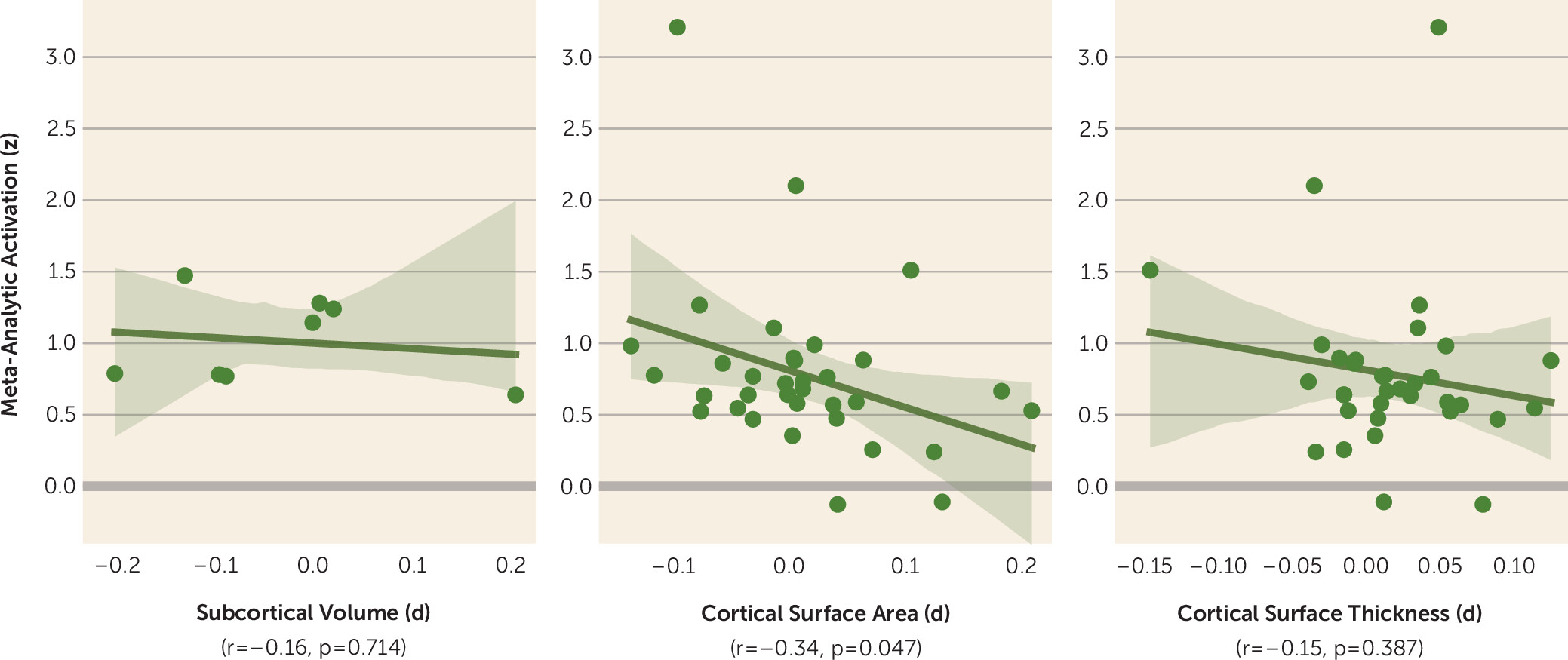
2), provides an opportunity to compare structural and functional differences region to region across the brain in phobia, as illustrated in
Figure 1.
The analysis summarized in the figure provides preliminary evidence for an overall negative relationship between function and structure, especially between cortical surface area and functional activation to emotion tasks in relation to phobias (r=−0.34, p=0.047). Regions for which individuals with a phobia tend to have a
smaller surface area are associated with greater
increases in blood-oxygen-level-dependent activation to emotion tasks. Exemplifying this is the anterior/mid-cingulate cortex, frequently reported as showing hyperactivation in participants with anxiety (
14,
15), which appears to show reduced surface area. One interpretation is that a reduced surface area might necessitate greater metabolic and functional activity to achieve the required processing demands of emotional stimuli, which are often biased in patients with phobias (
16). This relationship is of course tentative, given the broad comparisons of summary statistics between data sets (as opposed to subject-level metrics) and the cross-sectional nature of the correlations we present here. We also can’t rule out the possibility that this effect is driven by differences in how mega- and meta-analytic data points were adjusted for covariates otherwise associated with anxiety symptomatology and cortical morphology. Nonetheless, we must acknowledge there are likely inextricable structure-function relationships shaping cognition and behavior (
17) that have, for the most part, been neglected in anxiety research.
It is unclear whether anxiety-relevant brain function changes precede, and potentially drive, structural changes in the brain or vice versa. A fruitful avenue for exploring structure-function relationships in anxiety may come through closer multimodal examination of developmental trajectories. Hilbert et al. report that an association between brain structure and phobia was observed only in adults. Yet, there is a plethora of evidence demonstrating differential brain activation among children and adolescents with anxiety disorders (
18). Speculatively, such activation in the absence of structural differences suggests that function may be driving morphological differences during development. However, there is scant longitudinal evidence directly exploring this in pediatric anxiety, and it is nonetheless likely a bidirectional relationship. Understanding the developmental pathways that give rise to these causal influences between structure and function holds implications for neural malleability in response to treatment, underscoring the importance of age at which treatments for phobia start.
For future studies addressing structure-function hypotheses, we also emphasize strong consideration of the specificity of functional activation. We have previously demonstrated that naturalistic neuroimaging paradigms (i.e., suspenseful movies) can elicit differential—and even inverse—brain responses compared to traditional designs, such as using unpredictable shock and resting state (
8,
19–
21). This negative correlation between surface area and phobia-dependent brain activity to emotion tasks may thus differ depending on the type of anxiogenic stimulus. Yet, there is a clear lack of naturalistic neuroimaging data collected from individuals with pathological anxiety. We therefore encourage future investigations into anxiety to consider these paradigms, especially when contextualizing associations between structure and function.
In an age when the outlook for neuroimaging-derived biomarkers can seem bleak, Hilbert and colleagues’ work provides robust evidence for neuroanatomical differences among and between individuals with a phobia. Their evidence points to generally decreased subcortical volumes (except in the pallidum) and increased cortical thickness and surface area in volunteers with a phobia (compared with healthy control subjects). Moreover, phobia subtypes differed in measures such as frontal pole and medial orbitofrontal cortex thickness. However, contrary to a plethora of functional evidence, there was no indication of individuals with a phobia having altered whole-amygdala volumes. When contrasted with functional imaging data, there is a general negative association between cortical surface area and activation (r=−0.34), opening an avenue for exploring structure-function associations in anxiety. Going forward, we suggest that developmental and naturalistic neuroimaging methods will be crucial for understanding the interplay of brain structure and function in the context of anxiety.