Bipolar disorder is a serious and relatively common psychiatric disorder with a strong familial component, characterized by episodic disturbances in mood and cognition (
1,
2). Research on the neurobiology of bipolar disorder has increasingly incorporated the study of brain networks, or connectomics (
3–
5). Recent structural and functional imaging studies of bipolar disorder have revealed connectivity disturbances centered on key emotional and cognitive hubs such as the insula and inferior frontal gyrus (
6–
10). Such dysconnections produce a loss of integration between emotional circuitry and cognitive control networks, mirroring the hallmark affective and neurocognitive disturbances of the disorder (
5).
Interpretation of neurobiological differences in bipolar disorder is complex, as these differences may reflect biological risk factors, the consequences of the illness, or the impact of its pharmacological treatment (
11). Unaffected first-degree relatives of patients with bipolar disorder have an odds ratio of ∼7–14 of developing bipolar disorder (
12). Neuroimaging studies comprising young, unaffected bipolar disorder relatives are thus valuable, as they include high-risk individuals who will later convert to bipolar disorder but have not yet been exposed to the biological impacts of acute illness or psychotropic medication (
13–
15). Studies assessing white matter volume in individuals at high risk compared with control subjects have reported differences in the frontal gyrus (
16), internal capsule (
17), and corpus callosum (
18), mirroring gray matter changes such as cortical thinning (
19). Longitudinal studies show that neurobiological differences in those at high genetic risk evolve dynamically. For example, high-risk study participants show accelerated cortical thinning and volume reduction in the right frontal cortex, including the inferior frontal gyrus, lateral orbitofrontal cortex, frontal pole, and rostral middle frontal gyrus (
19).
Recent studies of high-risk cohorts have also employed connectomics, using diffusion-weighted MRI sequences to study the connectivity and integrity of white matter. Studies using local diffusion-derived indices (such as fractional anisotropy) have reported widespread changes (
20–
22) as well as localized disturbances in specific white matter tracts such as the uncinate fasciculus, corticospinal tract, cingulum, and corpus callosum (
23–
27). Whole brain tractography allows reconstruction of structural brain networks, a more direct study of connectomics in high-risk cohorts (
6). A recent study of young high-risk individuals found weaker structural connectivity in networks centered on the inferior frontal gyrus and insular cortex and stronger connectivity in a limbic network (
10). Notably, brain network differences in high-risk cohorts were distinct from those in established bipolar disorder, although both included regions involved in emotional processing and affect regulation, such as the insula, hippocampus, amygdala, and cingulate cortices.
The age range of bipolar disorder onset overlaps with the final stages of brain development and the accompanying reorganization of structural brain networks (
28). This reorganization happens in phases, shifting from sensorimotor loops in late childhood toward heteromodal and association cortex after adolescence (
29). These changes plateau in early adulthood, hence showing a nonlinear, age-dependent profile (
30), with behavioral correlates shifting from the acquisition of new skills toward the sophistication of behaviors and the maturation of cognitive control (
31,
32). Conceptual models of neuropsychiatric illnesses such as bipolar disorder suggest that they be framed as disturbances in this process, that is, as “neurodevelopmental miswiring” (
33).
Longitudinal studies are needed to disambiguate stable cross-sectional group differences from those that emerge in individuals during neurodevelopment or following illness onset. Only a small number of studies have investigated white matter changes in those at high risk of developing bipolar disorder. The Scottish Bipolar Family Study reported reduced fractional anisotropy across widespread brain regions over a 2-year period in individuals at risk of developing bipolar disorder who remained well, in those who developed major depressive disorder, and in control subjects. However, trajectories did not differ between groups (
22). Our group recently reported a significant increase in the prevalence of periventricular hyperintensities in high-risk young adults and control subjects over a 2-year period (
34). Again, no group differences in trajectories were evident.
Using whole-brain structural networks inferred from diffusion-weighted MRI, we studied connectomic changes over a 2-year interval, comparing young first-degree relatives of patients with bipolar disorder who did not have the disorder at baseline (the high-risk group) with control subjects from families without mental illness. Our cohort thus comprises adolescents and young adults who straddle the peak age of illness onset and who are themselves at high risk of bipolar disorder. We first report the linear and nonlinear reorganization of brain connectivity that occurs across the entire study cohort. We then report group differences in this reorganization, hypothesizing that these differences would be more pronounced in those high-risk individuals who experienced their first mood episode (a major depressive episode or a manic or hypomanic episode) between baseline and follow-up. We benchmark these changes against a group with established bipolar disorder, age-matched to our follow-up cohort. These analyses may help refine our understanding of neurodevelopmental processes in young people at high genetic risk of bipolar disorder and identify key protective factors and vulnerabilities.
Discussion
The peak incidence of bipolar disorder overlaps with the final stages of neurodevelopment in adolescents and young adults, a broad developmental phase that is associated with widespread longitudinal changes in structural connectivity. Consistent with the maturation of executive and cognitive control functions, connectivity in general tended to strengthen among central, midline, and rostral regions relative to posterior and central regions. Only very few changes were age invariant, consistent with well-established knowledge that cognitive and neural development occur in evolving waves of maturation (
30,
31,
52,
57). These longitudinal changes are consistent with other brain network studies, which report both increases and decreases in structural and functional networks during this critical neurodevelopmental phase (
28,
58–
62). Embedded in these shared connectivity dynamics was a significant group-by-time interaction, comprising a distinct subnetwork whose connectivity increased in our control cohort but weakened in the high-risk cohort, shifting toward a comparative cohort with established bipolar disorder. Intriguingly, our results provide marginal evidence that this effect is enhanced in those who experience a first episode of any mood disorder, particularly a bipolar-disorder-defining first manic or hypomanic episode. There is no comparable evidence of an effect associated with a prior mood episode or any new DSM-IV diagnosis. These results provide novel insights into the longitudinal dynamics of structural connectivity in individuals who are at high risk of bipolar disorder and an intriguing preview of personalized outcome prediction in this relatively common clinical scenario.
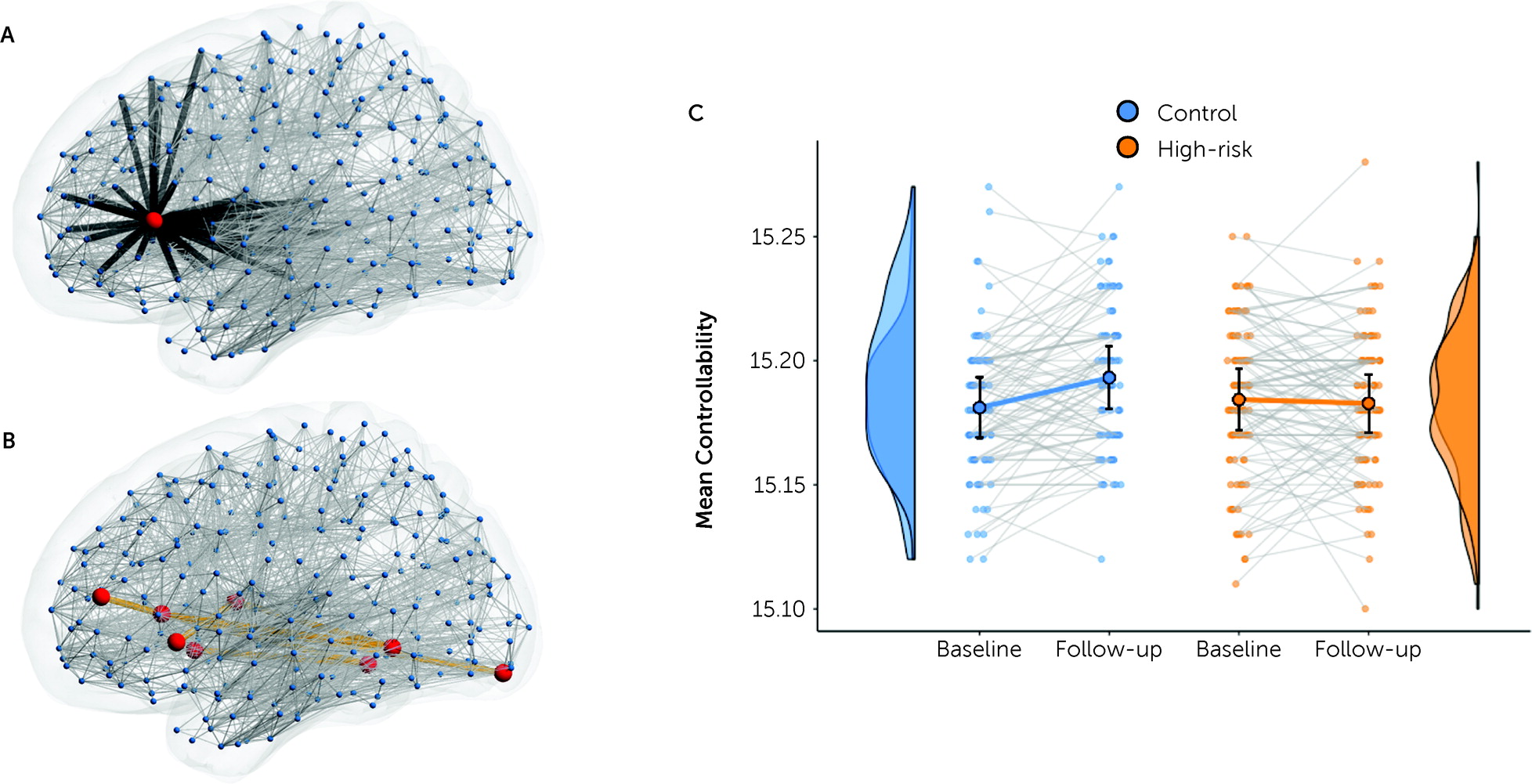
The structural network where development differed for our high-risk cohort comprises left inferior and lateral regions connected to the thalamus and bilateral posterior cortex. Of particular interest, this network includes the left insula and left inferior frontal gyrus—regions that have frequently been highlighted in cross-sectional studies of high-risk (
5,
7) and bipolar disorder (
63) participants, including baseline reports on the present population (
9,
10). These cortical hubs connect classic “limbic” regions such as the hypothalamus to cognitive control nodes, including the anterior cingulate and precuneus. Such connections underlie their integrative role in physiological, interoceptive, and executive functions (
64–
67) and their prominent role in anxiety and its dysregulation (
68). The broader network involves multiple brain systems in this network, including visual, default, control, and subcortical regions, also consistent with structural changes in bipolar disorder (
63).
Structural network changes identify candidate neurobiological markers of high genetic risk but do not, on their own, speak directly to the disturbances in affective and cognitive control that characterize bipolar disorder. Using computational methods, we found that the longitudinal network changes are associated with weaker network controllability, mirroring the emergence of affective dysregulation and disinhibition in bipolar disorder and its manic phenotype (
69). Although network controllability is influenced by network strength, it also reflects higher-order network properties, including local cycles within the target subnetwork and longer loops that permeate the broader connectome (
51). This loss of controllability thus shows how the subtle changes of edge strength within this network can have dynamic effects that propagate through the entire connectome, with brain-wide consequences.
There are several important caveats to our findings. Studying high-risk unaffected probands avoids the confounding effect of pharmacological treatment present in those with the established illness. Nonetheless, because we adopted an ecological approach (by not excluding those with any prior mood episode), a small number of participants in both groups were on pharmacotherapy. Current medications, current mood episode, and current mood state were not associated with the weighted strength of the group-by-time network. Other caveats of our study include the heterogeneous nature of high-risk cohorts, with some converting to the disorder while others remain well despite high genetic loading. Indeed, neurodevelopmental variability is a characteristic feature of healthy adolescent and young adult cohorts (
30). Moreover, developing algorithms for prognostic prediction relies precisely on variance in longitudinal outcome. The substantial variability across our high-risk cohort in clinical outcome and network effects also indicates the presence of protective factors and variability in genetic risk among first-degree relatives of persons with bipolar disorder.
While our study is well powered to achieve the primary objectives (of a group-by-time effect), only a small number of participants developed a first mood episode between baseline and follow-up. We found marginal evidence for a stronger network difference in those who developed their index case of mania during our study. Although these analyses are based on Bayesian methods (which avoid the need for a point-wise threshold for inference), this observation clearly requires replication in a sufficiently powered study. However, extrapolating the present rate of conversion from high risk to bipolar disorder at 2 years suggests that a total cohort size of up to 1,000 would be required to obtain a sample size of 40 high-risk-to-bipolar-disorder clinical converters over a 2- to 3-year time frame. Studies of this size invariably require pooling across multiple sites, introducing nuisance variance arising from site-specific imaging platforms.
Longitudinal studies are uniquely placed to disambiguate associations from mechanism (using temporal precedence) and support data-driven enrichment and prediction algorithms. However, longitudinal studies carry their own challenges. The time lag from study design and ethics approval through baseline to complete follow-up acquisition is typically longer than current funding cycles and also outlives most imaging-based innovations. Although we used advanced tractography pipelines, advances in diffusion MRI sequences since our study inception have improved the reliability and accuracy of the derived structural connectomes (
70). However, it is not possible to introduce these at follow-up in a longitudinal study because of the ensuing collinearity with follow-up characterization. The hardware, acquisition, and analysis pipelines were identical across both time points. Nonetheless, the role of the insula and inferior frontal gyrus is convergent with changes in other modalities (cortical thickness on structural MRI [
19]) as well as their role in cross-sectional studies of high-risk and established bipolar disorder (
5).
The acquisition of cognitive abilities mirrors neurobiological changes across developmentally active phases (
32), with changes in the sensorimotor cortex in childhood yielding to the structural reorganization of the heteromodal cortex in adolescence (
56). We found nonlinear changes (both slowing and accelerating) in a relatively small number of short, strong structural connections that were consistent across time points but unique between groups. The temporal stability of these “nonlinear edges” was lower in the high-risk group, suggesting greater neurodevelopmental heterogeneity, consistent with the variety of phenotypic outcomes. However, longitudinal changes in our data were (predominantly) linear, likely reflecting methodological choices imposed by the nature of our scan protocol. Recent developments in tractography, such as multishell acquisitions, structurally informed seeding, and anatomical filtering, may permit greater interrogation of nonlinear neurodevelopment in future longitudinal studies of high-risk individuals.
Persons with a first-degree relative with bipolar disorder often inquire about their own future risk of the disorder. Epidemiological studies show an overall odds ratio in the range of approximately 7–14 (
12), with the incidence peaking in the third decade of life. Prediction algorithms combining phenotypic, neurobiological, and genetic information are urgently needed to better stratify individual risk prediction, identifying those who might benefit from early intervention rather than the present “watch and wait” approach (5, 71). For example, in light of predictive genetic and imaging ascertainment, initiation of a mood stabilizer for a depressive episode might be preferable to an antidepressant alone in an “ultra-high-risk” first-degree relative. Here we show distinct neurodevelopmental effects in structural connectivity in this population, with a qualitatively stronger effect in those who did experience a manic episode between baseline and follow-up. Further work with greater numbers of participants and with more follow-ups is required to explore the potential of these observations and establish a possible clinical role.