Definition
NREM parasomnias are phenomena that emerge during arousal without complete awakening from sleep. Not included in DSM−5 is the momentary arousal with confusion, disorientation, and minimal movement without ambulation or autonomic activation known as confusional arousal. Sleepwalking (SW) represents motor activation during an abnormal arousal, usually from deep NREM sleep. Current diagnostic criteria in the DSM−5 include:
(A) repeated episodes of arising from bed during sleep and walking about with blank, staring face, unresponsiveness to others, and awakening only with difficulty.
There is typically (B) no or little recall of dream imagery, and
(C) none for the behavioral episodes. Events are associated with
(D) significant distress or impairment in social, occupational, or other areas of functioning,
(E) no influence of an exogenous substance, nor
(F), any coexisting psychiatric or medical disorders to explain the spells (
1).
Behaviors can vary from sitting up in the bed (confusional arousal) to full ambulation, and can include complicated behaviors such as walking, running, driving, and eating. These behaviors can occasionally result in sleep-related injury. When mental activity is recalled, it is described as dream-like visual imagery, both less detailed and less bizarre than traditional REM dream reports. Typically, these behaviors occur over minutes and more rarely over an hour or more during the early part of the sleep cycle, and emerging from the “deeper” stage N3 of NREM sleep, they may derive from any NREM stage. Note that sleep stage scoring was revised in 2007 and stages formerly designated as 3 and 4 of NREM sleep have been combined into the single stage now known as N3. Other stages of NREM sleep are now designated as N1 and N2 (
2).
This disorder of arousal, like those mentioned below, occurs when there is incomplete transition from NREM sleep to wakefulness. Phenomena that deepen sleep and enhance sleep inertia promote NREM parasomnias by impairing otherwise normal arousal mechanisms. These include sedative medications as well as increased homeostatic sleep drive such as those following sleep deprivation. Also, disorders causing repeated cortical arousals can lead to NREM parasomnias emerging during sleep fragmentation. These can include exogenous stimuli such as noises, or endogenous factors such as obstructive sleep apnea (OSA), periodic limb movements of sleep (PLMS), gastroesophageal reflux disorder, other physical illness, or full bladder, without any specific psychological meaning (
3–
8).
Sleep terrors (ST) are spells of abrupt “terror” arousals from sleep usually initiated with a scream. There is intense fear along with profound autonomic arousal including mydriasis, tachycardia, tachypnea, and diaphoresis. The remaining diagnostic criteria (B–F) are identical to those listed above for SW (
1). While not distressing to the amnestic individual, ST are very troubling to parents and other witnesses. As with SW, the individual is relatively unresponsive to attempts by others to provide comfort and consolation. Often, there can be accompanying motor behavior, including sitting up in bed and possible progression to SW. SW and ST, often occurring in combination, represent a continuum of events with exclusively motor or autonomic activation as polar examples (
3,
9,
10).
Various predisposing and priming factors frequently lead to SW either in isolation or in combination. Many individuals report that the frequency and severity of SW increases with stressful life experiences (
11,
12). Cases have been associated with migraine and thyrotoxicosis, fever, prior sleep deprivation, and central nervous system depressant drugs including alcohol. SW and ST both appear to demonstrate familial patterns. In one study, 80% of sleepwalkers and 96% of patients with ST could identify a family member who suffered from similar phenomena (
13). Central nervous system acting polypharmacy is often the setting for prolonged dangerous behavior such as sleep driving (
14,
15). Other examples include patients with OSA who are prescribed sedative-hypnotic medication to assist with continuous positive airway pressure (CPAP) compliance. A similar situation occurs often in patients with restless legs syndrome (RLS) misdiagnosed as having insomnia and subsequently treated with a benzodiazepine receptor agonist (BZRA) drug. As patients with RLS have a strong drive to ambulate, it is not unexpected that agents that suppress memory and executive function would lead to amnestic sleepwalking behaviors. When SW is associated with sedative hypnotics, it is of particular importance to reconsider the diagnosis for which the medication was originally prescribed. In these cases, patients may not have insomnia (for which the sedating agent was prescribed) but rather another disorder of sleep initiation such as RLS or a delayed circadian rhythm (
3,
9,
10,
15–
20).
Epidemiology and Biopsychosocial Underpinnings
Commonly beginning in childhood, SW peaks between 11 and 12 years of age. SW and ST generally subside in later childhood and adolescence but may continue into, and rarely arise during adulthood. The prevalence of disorders of arousal has been estimated at 1%−6.5% for ST and 5%−30% for SW in children and adolescents (
21–
23). It has been estimated that 2%−5% of adults may experience SW (
24–
26). A large systematic telephonic survey of individuals aged 15 years and older in the United Kingdom documents ST in 2.2% (2.6% for ages 15–24 and 1.0% for ages >65), SW episodes in 2.0% (4.9% for ages 15–24 and 0.5% for ages >65), and confusional arousals in 4.2% (8.9% for ages 15–24 and 1.4% for ages >65). In the same population, 2.0% of all respondents reported some violent behavior during sleep. Pure ST occurs more frequently in young children, arising in approximately 3% of children and less than 1% of adults (
24,
27,
28).
Historically, SW has been thought to be associated with psychopathology in adults (
29–
33). A study of 54 adults with SW/ST from a study of 100 patients with sleep-related injury based upon PSG, psychiatric interview, MMPI, Beck Depression Inventory (BDI), and SCL-90 (
34), includes current DSM–III axis I diagnoses in only 19/54 (35.2%) cases. Of these, 14/19 (37.6%) were mood disorders and 4/19 (21.1%) alcohol and/or substance use disorders. These did not appear to be temporally associated with the onset of parasomnias. MMPI data available for 36 of the 54 adults (66.7%) suggested possible personality disorders in only 12 (33.3%) (
5). A retrospective review of 11 cases of ST cites 7/11 patients reporting an influence of stress on their disturbance, though none had a diagnosis of panic disorder and the course of the sleep disorders did not overlap significantly with any lifetime mood or substance use disorders. The authors conclude that ST are “not simply symptoms of a psychiatric disorder” (
10,
12,
35). Recent epidemiological evidence suggests that there is some association between SW, major depression, and obsessive-compulsive disorders, though with no established causal relationship (
36).
Of particular interest to psychiatrists are cases of parasomnias related to the use of psychotropic drugs, in particular sedative hypnotics. One group of investigators noted a high frequency of SW and other amnestic complex behaviors among psychiatric patients taking BZRA medication (
15,
37,
38). These findings are consistent with other reports of abnormal nocturnal behavior induced by these drugs, especially zolpidem (
15,
18,
38–
46). These parasomnia behaviors can be prolonged and include amnestic nocturnal eating, sexual activity, and even sleep-driving. Not unexpectedly, these events have increased in parallel with the rise in the use of these sedative-hypnotic agents (
15). Note that it is the short duration of action hypnotics that seem to be associated with this phenomenon, whereas the longer duration benzodiazepines tend to suppress them. It is likely that these drugs dull the transition between sleep and wakefulness, permitting behavior that is unconstrained by executive function in susceptible individuals. Other drugs associated with SW and ST include the use of neuroleptics such as olanzapine (
47,
48) and quetiapine (
49), antidepressants including paroxetine (
50,
51), reboxetine (
52), and bupropion (
53), mood stabilizers and antiepileptics including lithium (
54–
56), topiramate (
57), valproic acid combined with zolpidem (
39), stimulants, and antihistamines, often in various combinations (
6,
7,
18,
58–
63). Two other medications associated with SW/ST are metoprolol (
64) and fluoroquinolone (
65).
Nathaniel Kleitman, the “father” of American sleep research, wrote, “all the characteristics of somnambulism underline the difference between wakefulness and consciousness” (
66). This idea is reinforced by a report of single photon emission computed tomography (SPECT) imaging during a polygraphically documented SW episode, demonstrating increased cerebral blood flow (CBF) in the anterior cerebellum (vermis) and posterior cingulate cortex when compared with quiet slow wave sleep. There were also large areas of frontal and parietal cortical decrements of CBF when compared with normal awake subjects. As anticipated by Kleitman, SW appears to represent a concurrence of increased motor activation and decreased executive function during incomplete, disordered arousals from sleep (
67).
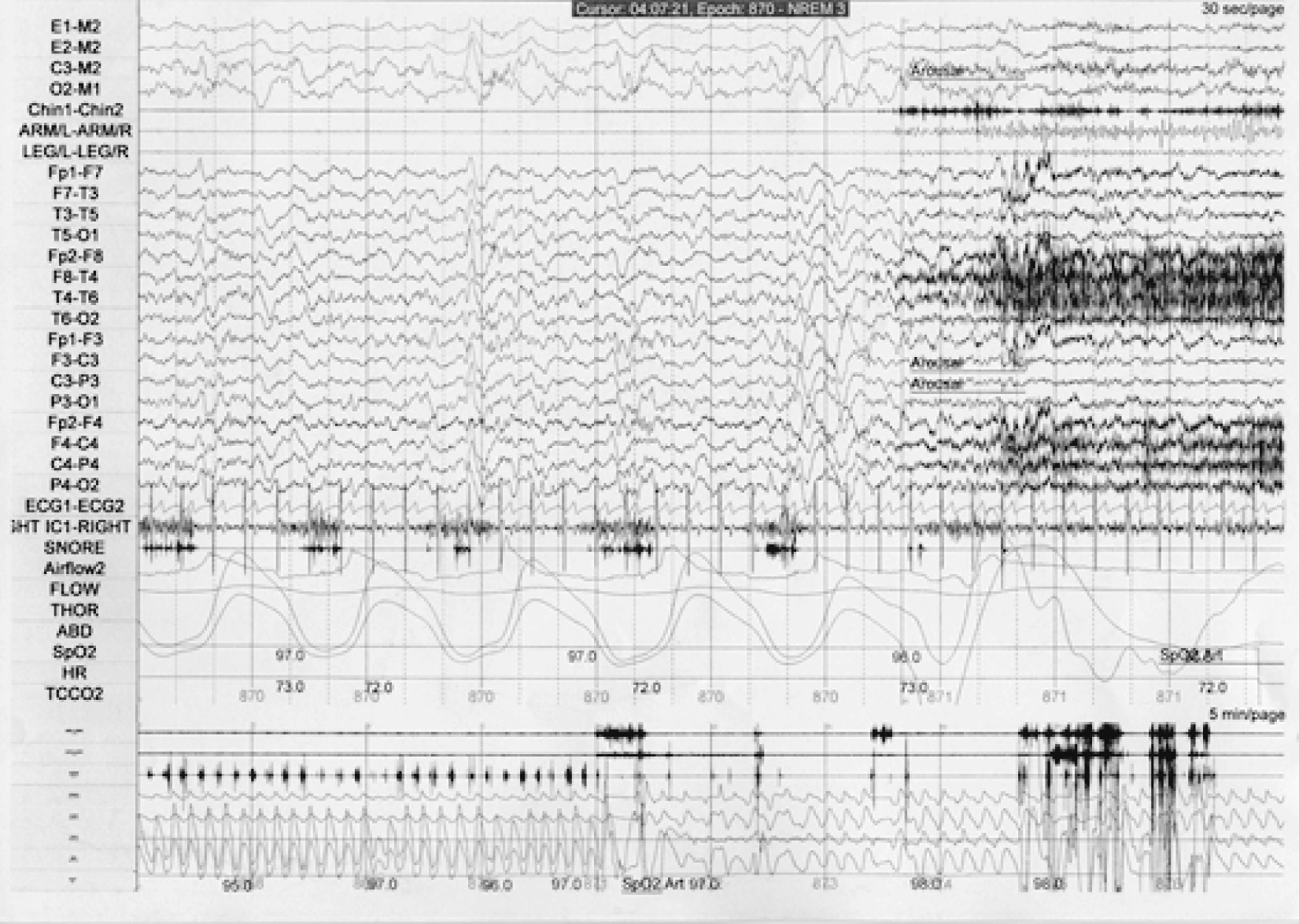
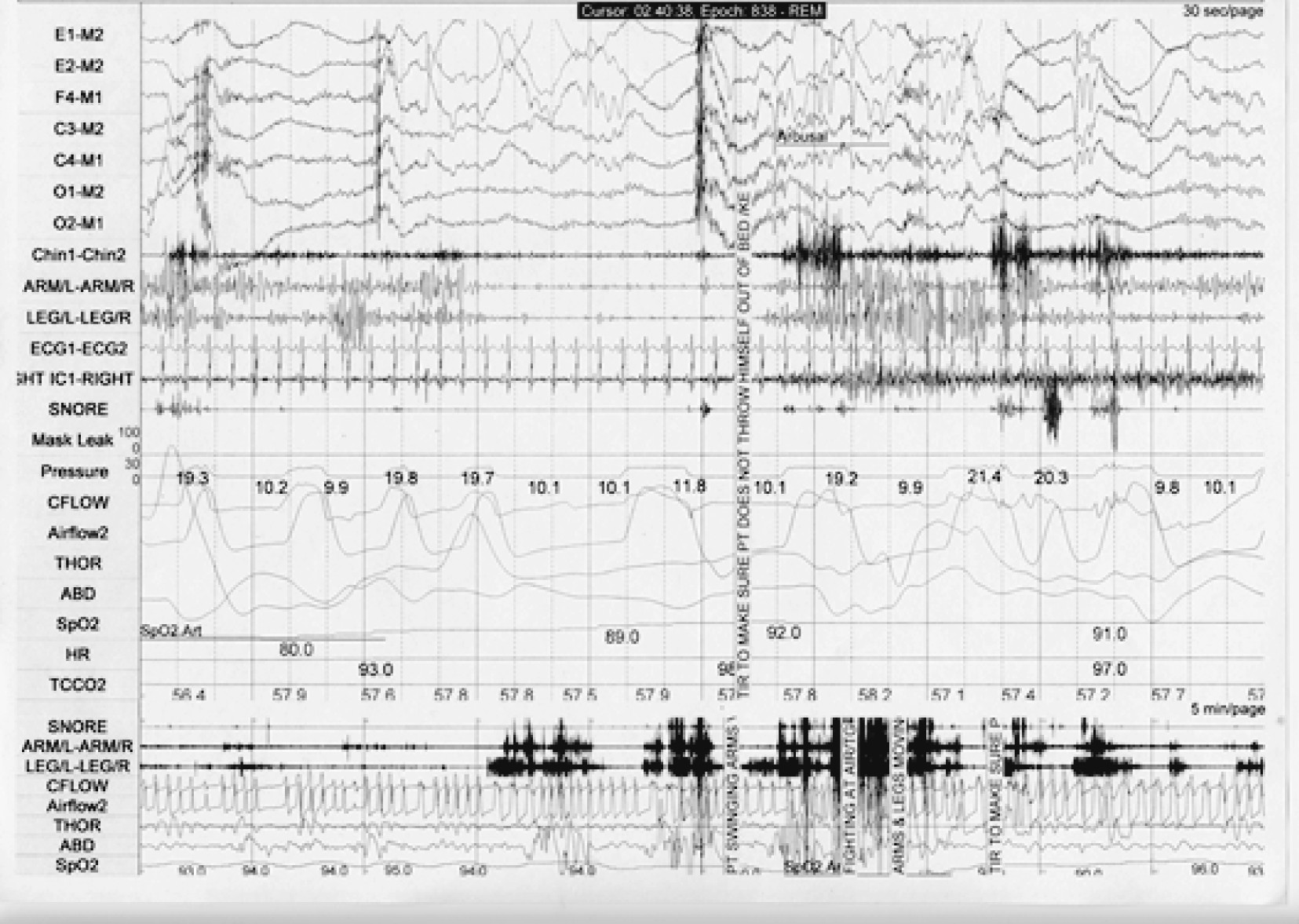
On PSG, there are few diagnostic markers of disorders of arousal in the absence of a spell. Bursts of slow EEG waveforms known as hypersynchronous delta activity are possible indicators of a drive to enhance depth of sleep but are not specific to these disorders (
68). Actual episodes of sleepwalking or sleep terrors are not observed very often during PSG. When they occur, they appear as abrupt arousals from non-REM sleep, typically but not exclusively from stages 3/4. With sleep terrors, there may be impressive tachycardia and tachypnea. Muscle activity often obscures the underlying EEG, which can demonstrate diffuse rhythmic delta activity, diffuse delta and theta activity intermixed with alpha and beta activity, and/or prominent alpha and beta activity. Hence, the EEG during episodes of disorders of arousal can show either the complete persistence of sleep, the admixture of sleep and wakefulness, or complete wakefulness in spite of the behavioral manifestations of a mixed state (
3,
9,
10,
69,
70).
Treatment and outcomes of Sleepwalking/Sleep Terrors
Sleepwalking and sleep terrors are often benign and self-limiting, especially in children, and may require no treatment beyond reassurance and attention to sleep hygiene. Attention should be paid to safety features of the sleep environment such as placement of dangerous obstacles, accessible windows and stairways, and other dangers. If falls from bed are possible, consider placing the mattress on the floor.
Reversing the comorbid conditions that predispose, prime, and precipitate parasomnias often dramatically diminishes nocturnal behaviors. Discontinuing offending agents will typical resolve the parasomnia, particularly if another underlying condition is identified and treated (
15,
17,
71). Identifying and reversing sleep-disordered breathing often results in a resolution of NREM parasomnias. In one study, 60 SW patients were studied with PSG, treated accordingly, and followed for 1 year. A high number (N=53) were diagnosed as having sleep-disordered breathing. The majority of patients had only a mild burden of disease, often not reaching the criteria for OSA, but instead reaching the criteria for upper airway resistance syndrome (UARS); they did not demonstrate daytime sleepiness. However, the results were striking. Only three patients dropped out of the study while of the remaining 50, all reported resolution of SW after treatment (42 reported CPAP, eight reported upper airway surgery). These dramatic results suggest that treatment of even mild, asymptomatic sleep-disordered breathing may result in the resolution of SW (
72).
In cases with a low risk of imminent injury, nonpharmacological therapy with clinical hypnosis may be preferred. This can be offered initially in combination with medication, which can be gradually withdrawn. Patients are instructed in the induction of a relaxed, meditative state, with visual imagery of quiet, restful sleep associated with comfort, safety, and reinforcement of a possible but minimally probable need for physical mobilization. With self-hypnosis utilized before retiring to bed, benefit has been reported in 20/27 (74%) patients who reported significant or very significant improvement (
73). In another study with similar techniques, 3/6 (50%) SW patients were greatly improved or spell-free after 18 months and likewise for 2/3 (67%) patients after a 5-year follow-up (
74). Progressive muscle relaxation training has also been utilized (
75). In children, the use of anticipatory awakenings (
76), clinical hypnosis (
77,
78), and a combination of acupuncture with medicinal herbs have been documented as helpful (
79).
When NREM parasomnias persist despite the resolution of inducing and exacerbating factors, pharmacological intervention is considered for disorders associated with the risk of injury or disturbance of the home environment. Evidence for all therapies for these disorders is limited to case reports, and rare controlled clinical trials with limited sample sizes. Complicating things further are some contradictory findings in the literature (
80). The earliest pharmacological agents offered to individuals at serious risk of injury were diazepam (
81) and imipramine (
82). Other agents considered to be anecdotally effective include other benzodiazepines, carbamazepine, doxepin, trazodone (
61), and melatonin (
83). All pharmacotherapy for parasomnias is currently utilized as “off label,” without formal FDA indications.
Intermediate and long-acting agents in the benzodiazepine class of sedative hypnotics (BZD) have become the most commonly reported pharmacological treatments for NREM parasomnias. Clonazepam has been reported to be effective in doses of 0.25–2.0 mg taken about 30–120 minutes before sleep. When given to 28/54 (51.9%) patients with SW/ST, it produced substantial benefit in over 80% (
5). In 1996, a series of 170 patients with mixed sleep disorders (69 with SW/ST) were treated with benzodiazepines, primarily clonazepam (N=136), and followed for clinical response (
84). The vast majority of all patients (86%) reported good control after an average follow-up of 3.5 years. We reported that clonazepam efficacy was sustained with low risk of dosage escalation. A separate clinical case series reported on six SW patients who were initiated on clonazepam. SW was suppressed in five of six patients (
85). The drug may work through the suppression of cortical arousals, but this is clearly paradoxical when considering the induction of amnestic nocturnal behaviors by the BZRA drugs (
15).
Conversely, a more recent report claims that clonazepam failed to demonstrate sustained efficacy in five SW patients. This investigation carefully excluded even subtle sleep-disordered breathing. After 1 year, all patients treated with clonazepam dropped out of the study and reported a persistence of SW (
72).
Distinct from BZD and BZRA drugs, a number of antidepressants have been reported to treat NREM parasomnias, most commonly ST. One report described two patients with a history of combined ST and SW, both of whom failed diazepam therapy but responded well to imipramine (a tricyclic antidepressant) (
82). The selective serotonin reuptake inhibitor (SSRI) paroxetine appears to be particularly effective in the treatment of ST. In one report, six patients had a significant reduction if not outright elimination of ST events. We suggested that SSRIs might be uniquely effective for ST through the effects of serotonin on terror centers in the midbrain periaqueductal gray matter (
86). In contrast to these successful ST cases, a more recent series of SW patients describes eight patients who were treated with various serotonergic agents and/or benzodiazepine. After a 1-year follow-up, all eight patients described a persistence of SW (
72). Further, there have been reports of paroxetine and sertraline allegedly inducing SW (
50,
51).