Delayed Sleep Phase Syndrome
Delayed sleep phase syndrome (DSPS) is characterized by sleep onset insomnia (without sleep maintenance insomnia), that causes excessive daytime sleepiness, specifically with difficulty waking up in the morning to attend to daily obligations such as work or school. The prevalence of DSPS is about 2% of the general population with a tendency to effect young adults and adolescents (
25,
26). In delayed sleep phase syndrome there is a high prevalence in both bipolar and unipolar depression. In one study using actigraphy, 62% of bipolar patients had delayed sleep phase when depressed compared with 30% of unipolar depression patients and 10% of healthy controls (
27).
DSM-V criteria indicate that the disorder can be classified into three subtypes. Episodic type patients have symptoms that last at least 1 month but less than 3 months. Persistent type patients have symptoms that last 3 months or longer. Recurrent type subtype patients have two or more separate episodes that occur within the space of 1 year. These patients present with insomnia at night and have difficulty waking up in the early morning. It can be further specified if the disorder is familial or overlaps with non-24-hour sleep wake syndrome.
Mutation in the
Per3 gene has been implicated in some families with delayed sleep phase syndrome (
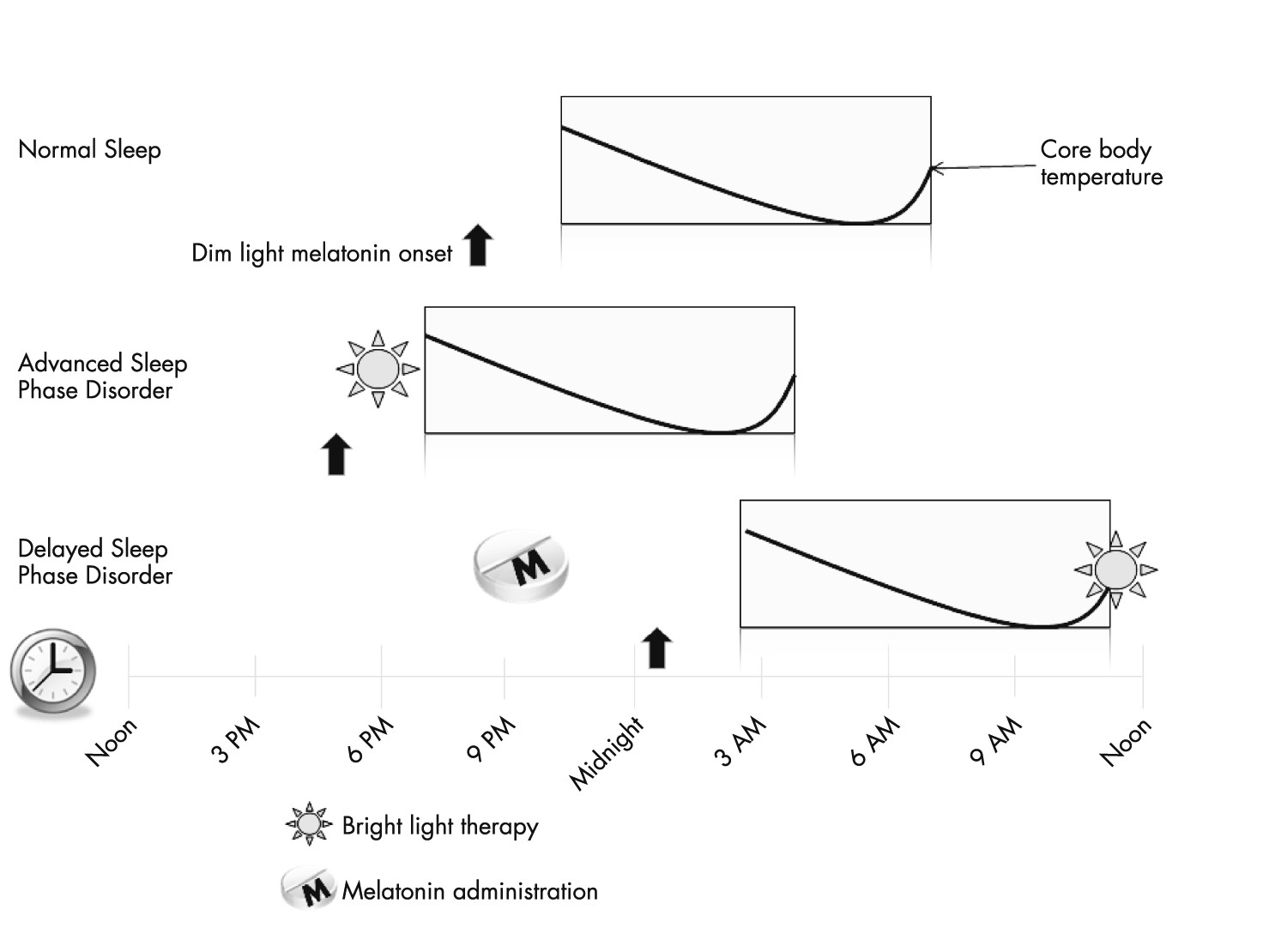
28). Patients fall asleep anywhere from 1:00 a.m. to 6:00 a.m. and have extreme difficulty waking up in the morning and can sleep as late as the early to midafternoon. Sleep logs or actigraphy can aid in the diagnosis and can be particularly helpful if a patient’s history is internally inconsistent or if the history is not concordant with the patient’s bed partner or parents. Even more objective data can be obtained through the use of salivary melatonin excretion or measurement of core body temperature. Patients with delayed sleep phase syndrome have their DLMO delayed by 2 hours or more and similarly, their core body temperature is delayed by the same amount of time when compared with the normal population (
29). Although these objective measures can be obtained, sleep logs are very predictive of these measures and are easier to acquire (
30).
Treatment for delayed sleep phase syndrome should be directed at adjusting the circadian rhythm through a combination of behavioral changes, evening melatonin, and bright light therapy. First, the patient should adhere to good sleep hygiene including the avoidance of caffeine in the afternoon and additionally avoid bright light in the evenings, including the light from computer screens, laptops, tablet PCs, and televisions. Melatonin should be given 3–6 hours prior to DLMO. In one study, melatonin doses at 0.3 and 3 mg were given to patients at variable times and compared with placebo to look for optimal dosing and the greatest phase advancement effect. The study found that the dose did not change the effect of the phase advancement, but the study did find that when melatonin was given 6.5 hours prior to DLMO, it produced the greatest phase advancement compared with other timings (
31). This timing corresponds to 13–14 hours after natural wakeup time (
32). Although the importance of this study was noted in the 2007 AASM practice parameters, and even though melatonin was recommended in the treatment guidelines, the AASM guidelines still indicated that the dose and timing of melatonin administration needed more study.
The importance of light therapy cannot be overstated in the treatment of DSPS. In the morning, bright light therapy in the form of 10,000 lux should be presented to further advance the circadian rhythm and core body temperature. Light will shift the circadian rhythm in a dose-dependent manner (
33) (see
Figure 2 and
Table 1). It should be presented shortly after awakening and care should be taken not to present bright light too early to a patient’s core body temperature minimum as this action could inadvertently delay sleep time even further as demonstrated by the following example.
A 16-year old boy who is a sophomore in high school presents with the inability to fall asleep until 3 a.m. and on weekends his preferential wakeup time is 11 a.m. In this example, DLMO is around midnight to 1 a.m., and the patient’s core body temperature minimum may be around 8 a.m. The patient’s parents forcefully wake the patient up every day at 6:30 a.m. with multiple alarms and by dragging him out of bed so the patient can wake up, have breakfast, and get to the bus to make it to school on time. By getting him up early, they expose him to bright sunlight from the outside world before his core body temperature minimum. This action causes light to actually delay the patient’s circadian rhythm even further. Parents may bring their child to a sleep clinic completely surprised that despite the fact their child wakes up early, and is tired or sleepy at school in the morning, he cannot fall asleep at a reasonable hour. They are more stunned to realize they may be the ones contributing to the underlying problem.
A final therapy worth discussing briefly for the treatment of DSPS is chronotherapy. There are no controlled clinical trials looking at its efficacy, but prescribing further delay of the patient’s sleep schedule can help the patient readjust the circadian clock. Since there are no trials, there is no consensus as to how long the therapy should be performed or how much additional sleep delay should be prescribed. For this therapy a patient is asked to stay up a few hours later every few nights until they eventually reach a socially acceptable sleep time. The difficulty in this practice is that patient would need to miss work or school, as they would need to sleep during most of the day until they finally reach a desired sleep time. However, for some patients who are already missing a substantial amount of work or school this therapy may be better than the alternative.
Non-24-Hour Sleep Wake Disorder
Non-24-hour sleep wake disorder is also referred to as “free-running circadian rhythm disorder,” or “non-entrained circadian rhythm disorder.” In this disorder the patient’s circadian rhythm is not entrained by the light and drifts toward the later hours. Classically, this disorder is seen in blind individuals but can occur rarely in sighted individuals. The ratio of men to women is 2.6:1 in both sighted and blind individuals. The disorder occurs because the natural circadian rhythm is not
exactly 24 hours, but is thought to be about 24.2 hours (
34). In sighted individuals it is postulated that the reason they develop this disorder is because they are less photosensitive than normal individuals (
35). In the absence of external queues the patient will fall asleep at later and later times every day. At times they will sleep during socially acceptable sleep times but only by coincidence when their clock is in alignment with social norms.
The clinical diagnosis is made primarily through careful history and sleep logs. The patient will complain of periods of insomnia during the evenings and hypersomnia during the day. This disorder greatly impacts a patient’s ability to work, perform in school, or even participate in social activities. Actigraphy can be very helpful as it shows a classical rightward shift of sleep-wake times.
For blind individuals the only treatment available until recently was melatonin. In sighted individuals the treatment is similar to delayed sleep phase syndrome with careful timing of light and melatonin. Patients must also be vigilant to try to maintain behavioral queues as well such as planned activities, timing of meals, etc. One recommended approach to melatonin treatment for free-running circadian rhythm disorders is to start with high dose melatonin (3 mg−10 mg) 2 hours prior to predicted DLMO nightly for 1 month. Once entrainment occurs over a period of 3–9 weeks, regular low dose melatonin should be continued to prevent a relapse (
36). With clinical trials now completed (
37), the drug Tasimelteon was recently approved by the FDA for the treatment of non-24-hour sleep wake disorder in the blind. Tasimelteon is thought to reset the master body clock in the SCN to synchronize the body’s melatonin and cortisol circadian rhythms with the day-night cycle.
Advanced Sleep Phase Syndrome
Advanced sleep phase syndrome (ASPS) is characterized by the inability to stay up in the evenings with early bedtimes and early morning awakenings. A patient’s chief complaint is the inability to stay awake and hypersomnolence in the early evening along with early morning awakenings. The onset is during middle age and some studies suggest that age is a risk factor for ASPS as circadian pacemaker phase advances with aging (
38). Two gene mutations identified in families with ASPS are
Per2 and casein kinase 1, delta gene (
26,
43), and when making a DSM-V diagnosis it should be indicated if there is a family history of this disorder. Patients are generally physically unable to stay up for social gatherings in the evening. APSP is less common than DSPS but the prevalence is likely underreported as many patients with this disorder tend to find jobs that allow them to maintain this lifestyle and do not seek treatment for it. In fact, many people with advanced sleep phase syndrome are rewarded professionally because they often show up very early to work. Patients with this syndrome usually fall asleep between 6 p.m. and 9 p.m. and will thus wake up from 2 a.m. to 5 a.m.
Again, detailed history is important to help distinguish ASPS from early morning awakenings due to depression, as this diagnosis can be made entirely from the sleep history. Sleep diaries and wrist actigraphy can also be helpful to make the diagnosis if more objective data are needed. The Horne-Ostberg questionnaire is also a useful tool to help identify morning-type versus evening-type individuals (
40). A polysomnogram is not necessary or indicated for the diagnosis of ASPS but it if it is to be done to evaluate for other sleep disorders, then care should be taken to perform the polysomnogram during the patient’s normal sleep time because early onset REM sleep may appear and the study may be limited by a low total sleep time.
American Academy of Sleep Medicine (AASM) guidelines indicate that evening bright light exposure is the only current treatment available for ASPS (
Figure 2). Theoretically, morning melatonin would help to delay an ASPS patient’s circadian rhythm, and although the AASM recommends evening melatonin for DSPS, they believe there is insufficient data to assess the safety and efficacy of timed morning melatonin administration for ASPS.
Shift Work Sleep Disorder
According to the Bureau of Labor Statistics, approximately 18% of the workforce is currently participating in shift work (
41). The prevalence of shift work disorder (SWSD) is estimated to be about 10% in shift workers (
42) but as high as 40% in nurses (
43). The ICSD-2 states that shift workers meet the following criteria: patients must (i) complain of insomnia or excessive sleepiness temporally associated with a recurring work schedule in which work hours overlap with the usual time for sleep; (ii) symptoms must be associated with the shift work schedule over the course of at least one month; (iii) sleep log or actigraphy monitoring for ≥7 days demonstrates circadian and sleep-time misalignment; and (iv) sleep disturbance is not better explained by another sleep disorder, mental disorder, a medical or neurological disorder, medication use, or substance use disorder. In a similar manner as DSPS, this disorder can also be subtyped as episodic, persistent, or recurrent by the same criteria.
Other symptoms of SWSD include chronic fatigue, malaise, mood disorders, decreased libido, dyspepsia, and other nonspecific complaints (
38). Shift work, even without shift work disorder, has also been linked to many other disorders, including an increased risk of breast cancer (
44), diabetes (
45), hypertension (
26), and depression (
46). In patients with shift work disorder, patients have an increased frequency of drowsy driving and an increased risk of work-related injury that is directly related to the amount of shifts worked (
44,
47).
The overnight shift causes the most severe symptoms but patients who work any shifts may also experience symptoms if the shift causes them to sleep at times that are misaligned with their endogenous circadian rhythm. Several factors may increase susceptibility to SWSD, including aging, presence of other sleep-related disorders, and the extent of daytime activities and commitments. On days when patients are not working their routine shift, attempts to sleep at socially normal times further worsen the misalignment of the circadian clock.
As with all sleep disorders, treatment first begins with creating good sleep habits, such as avoiding caffeine prior to scheduled sleep time, sleeping in a dark room, and maintaining routine sleep and wake times if possible. Proper nap timing before the beginning of an overnight shift has also been shown to be helpful in reducing symptoms of excessive daytime sleepiness (
48). When an overnight shift worker leaves his or her work to go home, avoiding bright light exposure by wearing sunglasses helps prevent circadian misalignment. Additionally, the use of modafinil or armodafinil has been shown to reduce symptoms of excessive daytime sleepiness during work (
49). A dose of 150 mg of armodafinil or 200 mg of modafinil has been shown to improve wakefulness when administered prior to the start of a shift (
Table 1) (
50). There have been multiple studies on the effects of melatonin. These studies show that sleep quality improves but melatonin does not help evening alertness (
51–
53). AASM guidelines indicate that the effectiveness of sleep quality has not been dose-responsive and some studies showed efficacy in as little as 1.8–3 mg of timed melatonin (
21). These guidelines also indicate that sedative hypnotics can be used to promote sleep during the day but carryover effects need to be addressed so the patient is not sleepy when scheduled to commute to work.
Jet Lag Disorder
Jet lag disorder is the most temporary of the circadian rhythm disorders. Although not every traveler will develop jet lag, most will experience some symptoms. It is a result of the timing of the circadian clock becoming misaligned from the timing of wake and sleep required in the new environment. Symptoms generally occur 1–2 days after travel and include complaints such as insomnia, excessive daytime sleepiness, fatigue, malaise, irritability, GI upset, and other nonspecific symptoms. Symptoms are dependent to the number of time zones changed. Westward travel is easier to adapt to than eastward travel because westward travel is corrected by delaying sleep onset (thus creating a greater homeostatic sleep drive). Eastward travel requires the traveler to advance their sleep time or attempt to fall asleep when their sleep drive is low. Older adults have a harder time adapting to jet lag than younger individuals (
54,
55).
A nonpharmacological approach that may minimize the effects of jet lag is timed light exposure and altering rest/activity times, although this approach has not been well evaluated. Following eastward flight, exposure to the local light-dark cycle usually accelerates adaptation after jet travel between 2 to 10 time zones. Additionally, shifting sleep/wake schedules earlier by 1 hour per day for 3 days prior to eastward travel may reduce jet lag symptoms. Westward travel is considered to be easier to adjust to than eastward travel; in anticipation of travel, travelers may delay their sleep/wake schedules in order to accelerate adjustment following arrival in the new time zone. For trips that are 2 days or fewer in either direction, advance preparation may not be necessary.
Currently, there are no approved pharmacologic agents for the treatment of jet lag. In a phase 3, double blind, randomized placebo-controlled clinical trial, armodafinil, at a dose of 150 mg, improved alertness demonstrated by mean sleep latency on a multiple sleep latency test and demonstrated improvement in jet lag symptoms through patient surveys (
Table 1) (
55). However, the FDA has not approved the use of this agent for the treatment of jet lag, and this study was performed after the AASM made its recommendations on the treatment of jet lag disorder. Melatonin has been studied extensively for the treatment of jet lag disorder. Many studies show melatonin is effective to reduce jet lag symptoms when taken prior to bedtime for eastward travel, but the dose is still debated (
21,
57). Two studies demonstrated melatonin’s efficacy when traveling westward for 12 time zones or more (
58,
59).