Worldwide, major depressive disorder imposes the largest burden of disease, measured in disability-adjusted life years, among mental, neurological, and substance use disorders (
1). Although major depressive disorder is amenable to pharmacotherapy, there remain major unmet needs for the treatment of depression, including the need for drugs with rapid therapeutic action and improved treatment response and remission rates for patients.
In recent years, considerable attention has been dedicated to the potential role of the glutamate system in antidepressant response. The most striking breakthrough in this field has been the discovery of the rapid antidepressant effects of ketamine, a primarily glutamatergic
N-methyl-
d-aspartate (NMDA) receptor antagonist. Subanesthetic doses of intravenous ketamine have been shown to elicit rapid, albeit transient, reductions in depressive symptoms in patients with unipolar and bipolar depression (
2). Although the antidepressant effects of ketamine become evident within a few hours or 1 day of a single infusion, the benefits generally disappear within 1 week (
3). To date, multiple randomized double-blind studies have shown that single ketamine infusions acutely reduce depressive symptoms when administered as monotherapy (
4–
7) and as an adjunctive medication (
8–
10).
More recent studies have examined whether repeated ketamine infusions can sustain the effects. Both twice-weekly (
11–
13) and thrice-weekly (
14–
17) administration schedules have resulted in sustained antidepressant effects as infusions are repeated. However, although repeated ketamine infusions may prolong antidepressant effects, relapse still occurs after cessation of infusions (on average, 18–19 days postinfusion) (
14,
15). Given the rapid relapse rates that follow ketamine infusion, the field requires the development of strategies that will permit a reduction in the frequency of infusions after response to acute treatment, with maintenance of the beneficial effects.
In the present study, the antidepressant effects of single, repeated, and maintenance ketamine infusions were characterized in a sample of patients with treatment-resistant depression. We hypothesized that the antidepressant effects of a single infusion of ketamine would be superior to an active control in a randomized double-blind crossover design; that increased response rates would be obtained with a course of repeated open-label ketamine infusions compared with those obtained with the single infusion; and that once-weekly maintenance infusions would prolong the antidepressant response obtained with acute ketamine treatment.
Results
Participants
Sixty-three individuals were prescreened for eligibility through consultation with a study physician. Of these, 46 signed consent forms and underwent formal screening (see the CONSORT diagram in Figure SF1 in the
online supplement). There were three screen failures. Forty-three participants met criteria for the intent-to-treat sample and received at least one infusion. Four participants withdrew during the course of the study. Participants’ baseline characteristics are summarized in
Table 1.
Single Ketamine Infusion
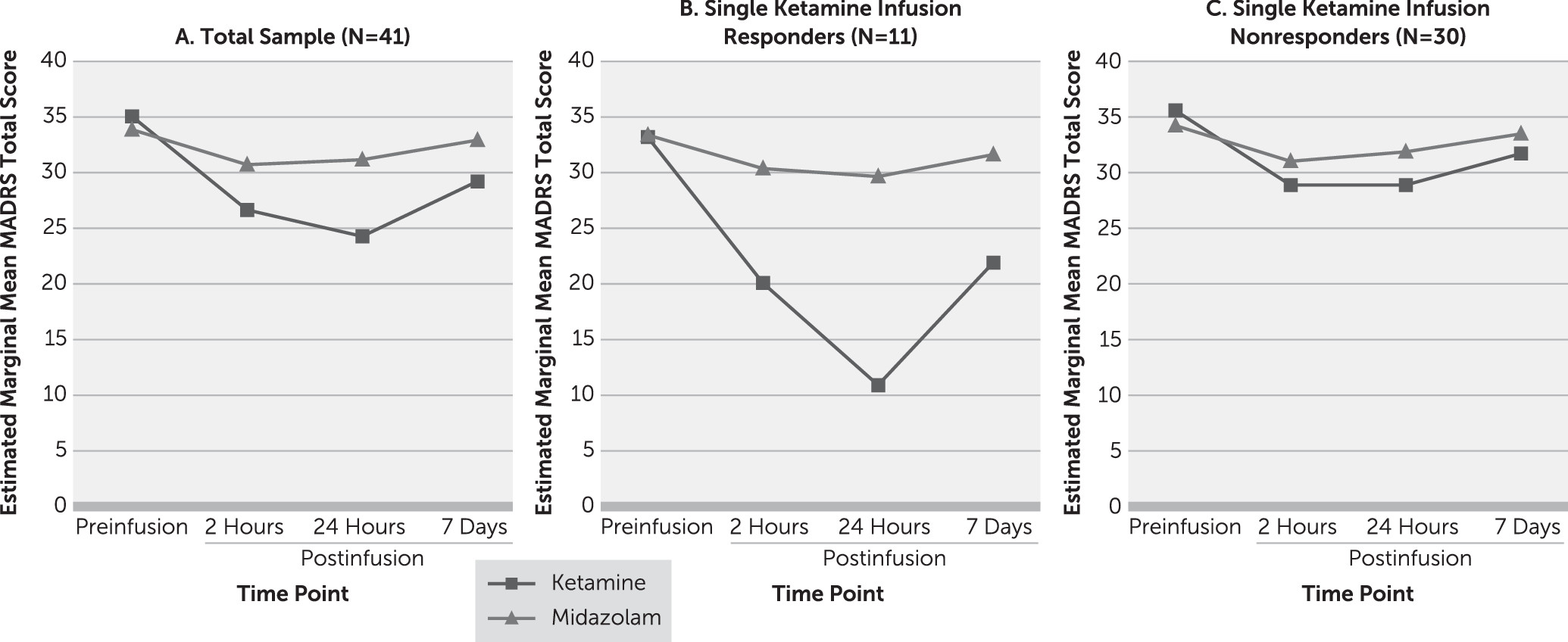
Forty-one participants completed the randomized double-blind crossover comparison of single infusions of ketamine and midazolam. Participants received the two infusions an average of 10 days apart (SD=6, range=7–36 days). There was no difference in the elapsed time between phase 1 infusions for participants who received ketamine first compared with those who received midazolam first (t=0.25, df=39, p=0.80). Using random-effects modeling, after adjustment for baseline MADRS score and order of drug administration, there were significant main effects for drug (F=8.84, df=1, 40, p<0.001) and time (F=30.77, df=3, 40, p<0.001) and a significant drug-by-time interaction (F=13.15, df=3, 40, p<0.001). Simple-effects analyses revealed that participants had significantly lower MADRS total scores at each postinfusion time point after ketamine infusion compared with midazolam infusion (
Figure 2A). At the a priori primary efficacy endpoint 24 hours after kettamine infusion, participants had a mean decrease of 10.9 points (SD=8.9) in MADRS total score relative to preinfusion scores compared with a mean decrease of 2.8 points (SD=3.6) with midazolam.
Examination of the model covariates revealed significant main effects for baseline MADRS total score (F=359.95, df=1, 37, p<0.001) and order of drug administration (F=4.24, df=1, 37 p=0.047), indicating carryover effects between phase 1 infusions. Although participants who received midazolam first had similar preinfusion MADRS total scores for both phase 1 infusions (mean, 35.4 [SD=5.6] compared with 34.9 [SD=4.5]; t=0.36, df=20, p=0.72), those who received ketamine first had slightly lower preinfusion scores at their second phase 1 infusion (mean, 34.6 [SD=4.1] compared with 32.6 [SD=5.6]; t=3.44, df=20, p=0.006).
Twenty-four hours after the single ketamine infusion, 11 participants (27%) met antidepressant response criteria, and two participants (5%) achieved remission. Single-infusion responders had a mean decrease of 22.3 points (SD=5.3) in MADRS total score (
Figure 2B) 24 hours postinfusion, and nonresponders had a mean decrease of 6.7 points (SD=5.6) (
Figure 2C). No participants met antidepressant response criteria with midazolam at any postinfusion time point.
Repeated Ketamine Infusions
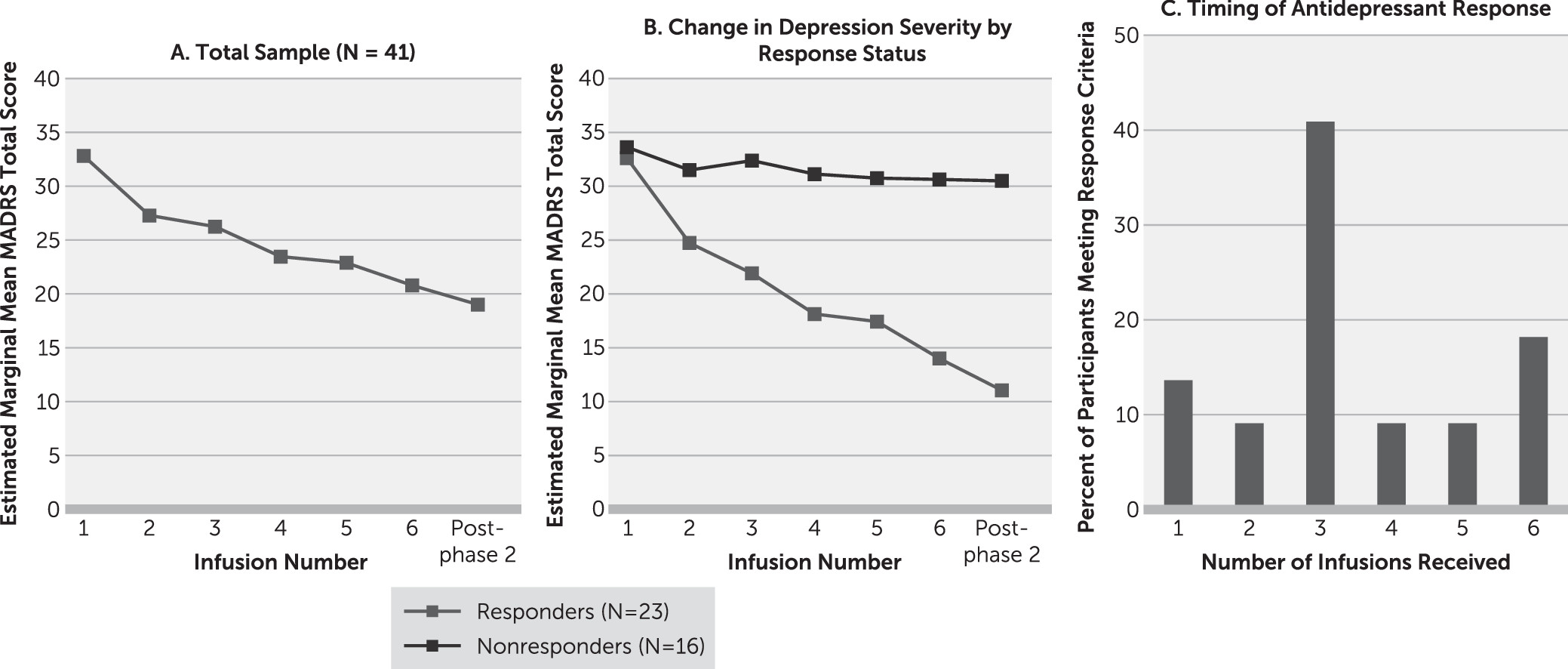
The primary outcome for phase 2 was change in MADRS scores over the course of six repeated infusions. A random-effects model that was adjusted for participant and depression severity at the start of phase 2 revealed a significant main effect for time (F=11.16, df=6, 39, p<0.001) (
Figure 3A). On average, participants’ MADRS total score decreased by 2 points with each infusion.
Of the 41 participants treated in phase 2, 39 completed the full course of infusions. At the post–phase 2 follow-up visit, 23 participants (59%) met antidepressant response criteria, and nine (23%) achieved remission. Responders had a mean decrease of 21.6 points (SD=5.8) in MADRS total score overall, and nonresponders had a mean decrease of 3.1 points (SD=5.7) (
Figure 3B). Responders included nine (82%) of the 11 participants who met response criteria after the single phase 1 ketamine infusion, and 14 additional phase 1 nonresponders. The median number of infusions participants needed to first meet response criteria was three. Seventy-seven percent of phase 2 responders received three or more infusions before meeting response criteria (
Figure 3C).
Maintenance Infusions
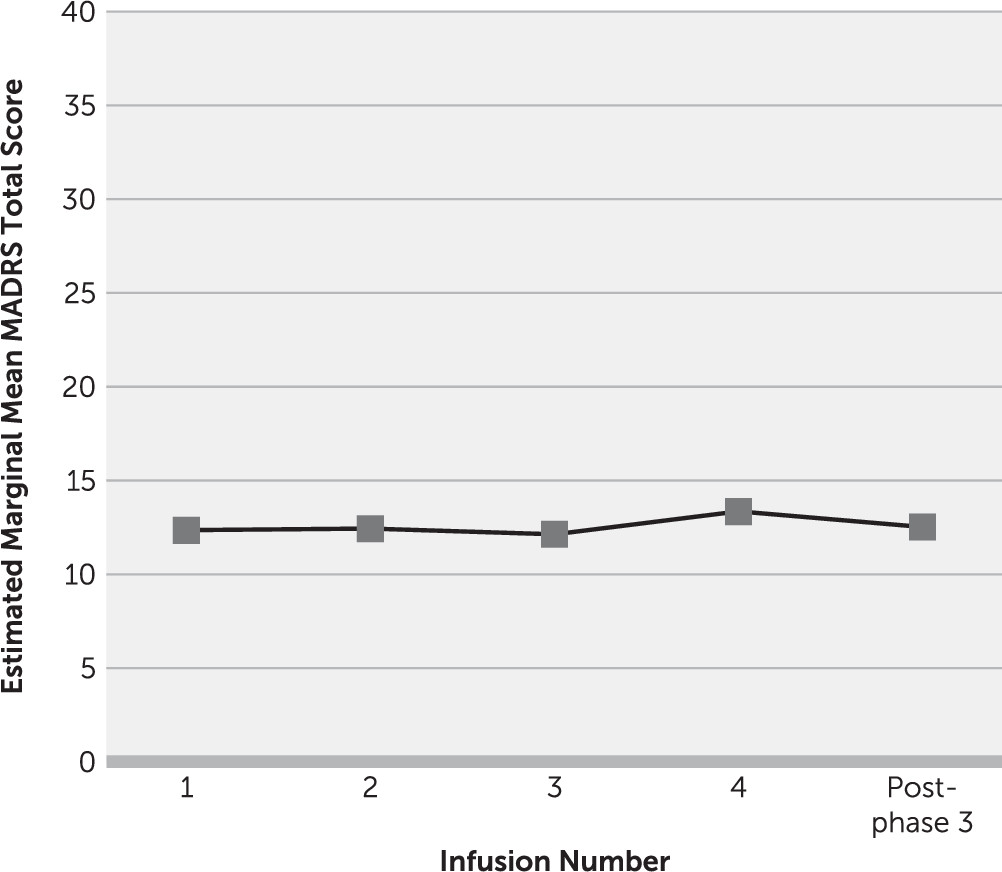
Participants who had at least a 50% improvement in MADRS scores after repeated infusions (N=23) continued to phase 3, the maintenance phase. A linear mixed model that was adjusted for the random effect of participant and phase-specific baseline depression severity revealed no main effect of time on change in MADRS total score during maintenance infusions (F=0.88, df=4, 22, p=0.49). This indicates no further change in MADRS score once ketamine infusions were reduced to once-weekly administration (
Figure 4). Examination of individual-level data revealed that 21 responders (91%) met antidepressant response criteria throughout maintenance infusions.
Self-Reported Depression Severity
Linear mixed models were used to evaluate participants’ self-reported change in depression severity by using QIDS-SR total scores. For phase 1, there were significant main effects for drug (F=6.60, df=1, 40, p=0.01) and time (F=6.76, df=3, 36, p=0.001) and a significant drug-by-time interaction (F=3.46, df=3, 37, p=0.03). Simple-effects analyses revealed that participants had significantly lower QIDS-SR total scores with ketamine compared with midazolam at 24 hours and 4 days postinfusion (see Figure SF2 in the online supplement). Consistent with the analyses of clinician-administered MADRS scores, there was a significant fixed effect of time on QIDS-SR total score in phase 2 (F=7.67, df=6, 39, p<0.001) (see Figure SF3 in the online supplement) and no effect for time in phase 3 (F=1.29, df=4, 22, p=0.30) (see Figure SF4 in the online supplement).
Safety Outcomes
No serious adverse events were reported during the trial. The most common side effects associated with ketamine were cardiorespiratory effects, numbness or tingling, dissociation, dizziness, and visual disturbances. Vital signs were continuously evaluated at each infusion throughout the study. Mean ketamine doses and overall changes to cardiorespiratory values were calculated for participants’ first ketamine infusion. The mean absolute dose of ketamine administered was 40 mg (range, 26–60 mg). During ketamine infusions, participants experienced transient elevation of blood pressure (maximum mean change, systolic, 25.3 mmHg; diastolic, 15.7 mmHg) and heart rate (maximum mean change, 10.2 bpm). On average, values returned to preinfusion levels at 24 minutes postinfusion (range, 5–40 minutes). No infusions were discontinued as a result of cardiorespiratory effects, and no rescue medications were administered during the study.
The dissociative effects of ketamine were formally assessed in a subgroup of study participants (N=22). A paired t test comparing participants’ preinfusion change with their immediate postinfusion change in CADSS total score during their phase 1 infusions revealed significantly higher CADSS scores with ketamine compared with midazolam (t=5.70, df=21, p<0.001). The dissociative effects associated with ketamine returned to baseline levels by 2 hours postinfusion (t=1.70, df=21, p=0.10). In phase 1, participants’ dissociative side effects (change in CADSS score) during the ketamine infusion were significantly correlated with antidepressant response at 24 hours postinfusion (change in MADRS total score, Pearson’s r=–0.46, p=0.03) (see Figure SF5 in the online supplement). Comparison of change in CADSS scores before and after participants’ first ketamine infusion (mean=18.0, SD=14.3) and their final ketamine infusion in phase 2 (7th infusion overall; mean=4.6, SD=7.3) revealed a decrease in dissociative side effects with repeated infusions (t=4.06, df=19, p=0.001).
Although drug craving was not formally assessed during the study, there were no spontaneous reports of craving or drug-seeking behavior among the participants during the trial nor in any participants seen since in follow-up.
Discussion
The results of this clinical trial confirm that subanesthetic ketamine infusions can provide safe and efficacious reduction in depressive symptoms in patients with treatment-resistant depression. Major novelties of this study include that it is the first trial, to our knowledge, to use a randomized double-blind crossover design to elicit superior antidepressant effects with a single infusion of ketamine compared with a psychoactive control. The study also showed restoration of antidepressant response with ketamine after relapse of depressive symptoms, allowing for the first direct comparison of response rates for single and repeated infusions, and evidence of a lack of tachyphylaxis to the benefits of ketamine. Additionally, this is the largest study to date to report maintenance of antidepressant effects in responders when the frequency of infusions was reduced to once weekly. Together, these findings help further inform the use of ketamine in clinical practice.
Consistent with previous studies, the single ketamine infusion had maximal antidepressant effects at 24 hours postinfusion, which abated within 7 days. Randomized clinical trials have consistently and repeatedly demonstrated the rapid antidepressant effects of single infusions of ketamine compared with saline (
4,
6,
8–
10) and midazolam (
5,
7). What is unique to the present study is the use of an active placebo control with a crossover design. All participants received both study medications, and none exhibited a clinically meaningful response to midazolam. This lack of placebo response and the relatively low overall antidepressant response to the single ketamine infusion (27%) highlight the illness severity and treatment resistance among the study participants. It may also suggest enhanced reliability of the findings, because the rapid reduction in depressive symptoms observed in single-infusion responders is more likely attributable to the ketamine infusion than to other factors associated with study initiation in a highly supportive clinical setting. Other studies that have employed a parallel design and reported higher response rates to single ketamine infusions have also reported notable response rates with midazolam, ranging from 11% (
7) to 28% (
5). The response rate with midazolam in the present study was 0%. These findings suggest that the response rates with ketamine reported in previous studies may need to be adjusted for placebo response.
Because all participants had a relapse of depressive symptoms before entering the repeated administration phase, it was possible to compare antidepressant response to single and repeated infusions in the same individuals. Results revealed a doubling of the antidepressant response rate with repeated infusions and no evidence of tachyphylaxis. Nine of 11 participants who responded to the single infusion also responded to repeated infusions despite relapsing in between. This suggests that ketamine may differ from other medications used to treat mood disorders, with which patients may fail to achieve a response after relapse if a medication is discontinued prematurely (
27).
Overall, more than half of the study participants met response criteria, and almost a quarter achieved remission by the end of the course of six repeated ketamine infusions administered over 2 weeks. The antidepressant effects of repeated infusions were cumulative, with depression severity further decreasing with each infusion and an increasing number of participants meeting response criteria as infusions continued. The prevalent late response to serial ketamine administration is consistent with the findings of Shiroma et al. (
16), with more than three-quarters of study participants requiring three or more infusions before meeting response criteria. This indicates that individuals who initially fail to respond to a single ketamine infusion may respond to repeated administration.
The goal of the final phase of the trial was to test, in all 23 ketamine responders (the largest group, to our knowledge, examined with maintenance infusions to date), whether antidepressant response could be prolonged when the frequency of ketamine infusions was reduced. Results clearly demonstrated that weekly maintenance infusions were sufficient to maintain the antidepressant effects obtained with repeated infusions.
Although there was no formal follow-up of study participants after the completion of phase 3, 10 study participants were subsequently enrolled in a psychotherapy study led by one of the authors (J.T.). Participants who enrolled in the secondary study within 2 weeks of completing the ketamine trial maintained their antidepressant response (N=6). However, participants who enrolled 3 or more weeks after their final ketamine infusion relapsed, and each presented with severe depressive symptoms at the initiation of the psychotherapy study (N=4). These findings, although descriptive in nature, suggest that continuation of ketamine infusions is necessary to sustain the antidepressant benefits even after a course of successful maintenance infusions. Wilkinson et al. (
13) recently provided preliminary evidence of the safety and tolerability of long-term ketamine administration, which mirrors our own clinical experience. Nevertheless, the prolonged use of ketamine warrants further study.
Overall, ketamine infusions were safe and well tolerated by study participants, with only transient side effects. Dissociation was among the most commonly reported side effects experienced. There have been reports of an association between dissociative side effects and antidepressant response to ketamine (
28,
29). In the present study, although participants’ initial antidepressant response to ketamine was associated with dissociative experience, dissociation decreased with repeated infusions despite increasing therapeutic benefits. This suggests that dissociative side effects do not entirely account for the antidepressant efficacy of ketamine, and additional research is required to further elucidate the relationship between these variables and to clarify the mechanisms underlying the antidepressant effects of ketamine.
Despite the strengths of this study, it has several limitations. These include the absence of dissociative side effects with midazolam, which has implications for the integrity of the blind in the crossover comparison in phase 1. Although the use of an alternate active control that has dissociative effects comparable to ketamine without antidepressant properties would have been ideal, no such drug has been identified to date. Another consideration is that the repeated and maintenance phases of the trial were open-label with no active control. Ketamine was shown to be superior to midazolam in this sample through the single-infusion comparison. The goal of phase 2 was to test reinstatement of the antidepressant response to ketamine after relapse. The use of a control during phases 2 and 3 would not have been successful, because after patients’ exposure to both midazolam and ketamine in phase 1, nearly all would be able readily to discriminate ketamine because of its mild but clear dissociative effects, thus compromising any future blinding of study medications. Furthermore, the fact that the 16 individuals who did not meet response criteria with repeated infusions showed virtually no decrease in MADRS score over time suggests that there was no interference from the clinical setting. Moreover, although these results are based on a modest sample size, the study was sufficiently powered to detect outcomes with the crossover design. Finally, given that participants maintained their concomitant medication regimen throughout the trial, it can only be concluded that ketamine is an effective adjunctive treatment, and it remains to be determined whether the same effects would be observed when ketamine is used as a monotherapy.
A recently published consensus statement recommended the use of ketamine in treatment-resistant depression provided that safety measures are in place and adequate psychiatric follow-up is available (
2). Within the field, however, an effective strategy to maintain antidepressant response in patients after cessation of infusions remains elusive. Although this study provides evidence of sustained antidepressant effects with once-weekly maintenance infusions, a future research goal will be to determine the degree to which the frequency of infusions can be decreased while maintaining response. Additionally, there is a need to directly compare the efficacy of ketamine to treatments with demonstrated antidepressant properties, such as ECT; such research is currently being conducted by our team. Ketamine shows great promise in providing rapid therapeutic action and improving response and remission rates in treatment-resistant depression. Although research into the mechanisms underlying the antidepressant effects of ketamine continues, this and future clinical studies can provide essential information on effective administration strategies that will permit sustained therapeutic benefits for patients.