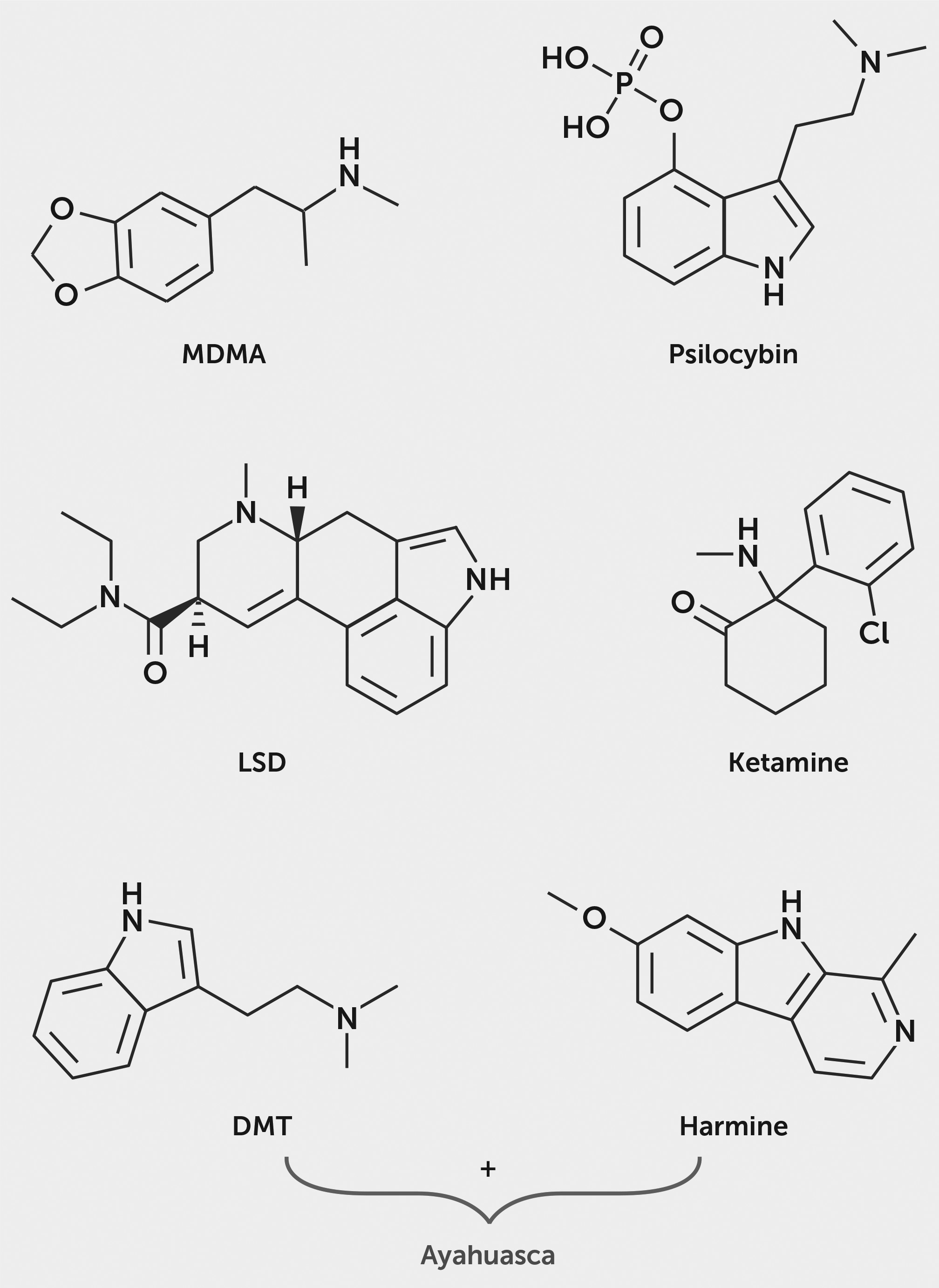
Psilocybin.
Psilocybin is a plant alkaloid derived from tryptamine precursors and found in a variety of mushroom species (
9). It has been used by native peoples of Central and South America within a sacramental context for centuries to facilitate spiritual experiences (
10). In the 1950s, psychedelic mushrooms were introduced to Western culture when amateur mycologist R. Gordon Wasson and his wife, pediatrician Valentina Wasson, published a story in
Life magazine describing their experience with psilocybin during participation in a Mazatecan ceremony in Mexico. The psychoactive compounds psilocybin and psilocin were first isolated from the mushroom species
Psilocybe mexicana through collaborative research by mycologist Roger Heim and Albert Hofmann and his colleagues at Sandoz Laboratories (
1). After determining the molecular structures of these compounds, Sandoz began the synthetic chemical production of psilocybin, eliminating the previously required cultivation of mushrooms (
1).
Psilocybin is actively metabolized to psilocin, a serotonin transporter inhibitor and 5-HT
2A receptor partial agonist with <40% activation efficacy; it also binds to the 5-HT
2C, 5-HT
1A, and 5-HT
1B receptors, with binding affinities in descending order (
11,
12). When taken at high doses (0.3–0.6 mg/kg), it can cause mild to profound changes in sensory perception, including synesthesia, euphoria, sensory illusions, and auditory and visual hallucinations. These effects are dose dependent and last 3 to 6 hours (
13–
15). Unpleasant effects can include feelings of a seemingly “unending experience,” as well as nausea, vomiting, and transient headaches (
16–
18).
Systematic investigation into psilocybin began in 1962, when Walter Pahnke and Timothy Leary conducted the “Marsh Chapel Experiment,” also known as the “Good Friday Experiment” (
19,
20). In this randomized controlled trial, Protestant divinity student volunteers (N=20; 10 per group) received psilocybin or a placebo (niacin) to evaluate the potential entheogenic properties of psilocybin. While the active and control drugs had differing physiological properties that likely challenged the blinding of the experiment, measurement of participants’ responses with an eight-category scale for mystical experiences confirmed the hypothesized effect of psilocybin (p<0.05).
Leary and colleagues also conducted the “Concord Prison Experiment” to determine whether psilocybin-assisted group psychotherapy could reduce rates of recidivism after a period of incarceration (
21). In this open-label study, prison inmates (N=32) participated in two psilocybin-assisted group psychotherapy sessions, each with a dose of 20–70 mg, followed by a series of psychotherapy sessions. Despite initial reports by Leary that psilocybin significantly reduced rates of recidivism, a later reanalysis by Doblin found that the recidivism rate of the experimental group was not significantly lower than that of the general prison population (
20,
22).
Recently, there has been a resurgence in psilocybin research in the United States and Europe in the treatment of refractory mood disorders, refractory obsessive-compulsive disorder, end-of-life anxiety, and tobacco and alcohol use disorders. Carhart-Harris et al. (
23) conducted an open-label pilot study evaluating the feasibility and efficacy of psilocybin-assisted psychotherapy for patients (N=12) with moderate to severe depression (defined as a score >17 on the Hamilton Depression Rating Scale [HAM-D]) and treatment-refractory depression (no improvement after trials of two different classes of antidepressant medication lasting at least 6 weeks within the current episode). Participants were given two oral doses of psilocybin in association with psychotherapy sessions, 7 days apart; they received a low dose (10 mg) of psilocybin at the first session and a higher dose (25 mg) at the second session. During the psilocybin sessions, therapists used a nondirective, supportive approach. All assessment measures were performed at baseline and at 1 week and 3 months after the second psilocybin-assisted psychotherapy session. The primary measure for efficacy was the Quick Inventory of Depressive Symptomatology (QIDS). QIDS depression scores were significantly decreased from baseline to 1 week and 3 months after treatment. The mean change in QIDS score was −11.8 (SD=4.9; p=0.002) at 1 week and −9.2 (SD=6.0; p=0.003) at 3 months. Secondary measures included the HAM-D and the Beck Depression Inventory (BDI). At the 1-week follow-up, categorical remission (defined as a score ≤9 on the BDI) was achieved by eight patients (67%). At the 3-month follow-up, categorical response (a 50% reduction in BDI score) was achieved by seven patients (58%), and five patients (42%) remained in complete remission.
In the same sample, functional MRI (fMRI) scans were performed at baseline and again the morning after the high-dose psilocybin-assisted psychotherapy session (
24). One day before and 1 day after their psilocybin sessions, patients were shown images of faces with fearful, happy, or neutral expressions selected from the Karolinska Directed Emotional Faces set. Patients who received psilocybin showed increased amygdalar responses to fearful compared with neutral faces 1 day after treatment, and this response predicted positive clinical outcome 1 week later. Heightened amygdalar activity following psilocybin administration was interpreted as evidence of a different antidepressant mechanism of action than that of patients treated with selective serotonin reuptake inhibitors (SSRIs), who have shown diminished amygdalar response to emotional stimuli. Further fMRI research has demonstrated that psilocybin acutely disrupts default mode network connectivity, inducing temporary neuroplastic states that may make an individual more susceptible and receptive to cognitive functions and content accessed with coadministered nondirective supportive psychotherapy (
25,
26).
Mood and adjustment disorders comorbid with cancer diagnoses are debilitating and are associated with poor clinical outcomes (
27). Grob et al. (
28) performed a randomized clinical trial (N=12, 11 of them women) investigating the safety and efficacy of psilocybin for the treatment of anxiety in patients with advanced-stage breast (N=4), colon (N=3), ovarian (N=2), peritoneal (N=1), or salivary gland (N=1) cancers or multiple myeloma (N=1). Each subject acted as his or her own control and had two treatment sessions in random order spaced several weeks apart: one session with a moderate dose of psilocybin (0.2 mg/kg) and the other with active placebo (niacin 250 mg). While there was no significant change in the self-reported State-Trait Anxiety Inventory (STAI) state score, STAI trait scores were significantly decreased at follow-up assessments 1 month (p=0.001) and 3 months (p=0.03) after the second treatment session. BDI scores did not change from baseline (1 day before placebo administration) to the 2-week follow-up assessment, but they dropped significantly by 1 month (p=0.05) and remained significantly different at 6 months (p=0.03).
A similar but larger double-blind randomized crossover study by Griffiths et al. (
18) (N=51) investigated the effects of psilocybin, administered in two sessions, on depression and anxiety syndromes in patients with terminal cancer who also had a DSM-IV diagnosis of an anxiety or mood disorder. The primary cancer types were breast (N=13), upper aerodigestive tract (N=7), gastrointestinal (N=4), genitourinary (N=18), hematologic malignancies (N=8), and other (N=1). Participants were excluded if they were taking psychoactive prescription medications (e.g., SSRIs, monoamine oxidase inhibitors, benzodiazepines). During the psilocybin sessions, participants received a high dose (22 mg/70 kg) or a low dose (1 mg or 3 mg/70 kg) of psilocybin, with the low dose serving as an active control. Participants were crossed over to receive the alternative dose in a second session 5 weeks later.
Before the first psilocybin session, participants met with study monitors to discuss “meaningful aspects” of their lives. During dosing sessions, therapists provided a supportive presence and encouraged participants to “trust, let go, and be open” to the experience, but otherwise were nondirective. The data showed that high-dose but not low-dose psilocybin produced large and significant decreases in depression and anxiety symptoms after 5 weeks, and this effect persisted through 6-month follow-up. A clinically significant response was defined as a decrease of ≥50% in score on the GRID-HAM-D-17 or the HAM-A relative to baseline, and scores below threshold level (≤7) defined symptom remission on each measure. The 6-month response rate was 78% for depressive syndromes using the GRID-HAM-D-17 and 83% for anxiety syndromes using the HAM-A; remission scores were achieved by 65% of participants on the GRID-HAM-D-17 and by 57% on the HAM-A.
A double-blind placebo-controlled (using niacin) randomized controlled crossover study by Ross et al. (
29) (N=29) evaluated the efficacy of a single high dose of psilocybin (0.3 mg/kg) in conjunction with medication-assisted psychotherapy in patients with cancer-related anxiety and depressive symptoms as measured by the Hospital Anxiety and Depression Scale (HADS), with subscales for anxiety (HADS-A) and depression (HADS-D). Approximately two-thirds of the patients had advanced (stages II–IV) cancer, and the types of cancer included breast or reproductive (59%), gastrointestinal (17%), hematologic (14%), and other (10%). The BDI and the STAI state and trait scales were also administered at baseline and at regular intervals during the study. After 7 weeks, the placebo group was crossed over to psilocybin and the active psilocybin group to placebo. Medication-assisted psychotherapy included preparatory psychotherapy, medication dosing sessions, and postdosing integrative psychotherapy. During medication-assisted psychotherapy sessions, participants were encouraged to lie comfortably on a couch, to wear eye shades, to listen to preselected music, and to direct their thoughts toward their internal experience. Two study therapists, typically one male and one female, were present and available for psychological and medical support throughout the duration of the experimental sessions.
There were significant reductions in all of the primary measures (HADS total, HADS-A, HADS-D, BDI, STAI state, STAI trait) in the psilocybin group compared with the control group immediately after the experimental session, and these reductions were maintained until crossover of the control group at week 7. The psilocybin-first group had significant within-group reductions compared with baseline in anxiety and depression at all six time points, including the final time point at 26 weeks after dosing. Before being crossed over to psilocybin, the placebo-first group had no sustained significant reductions on any of the primary measures. Immediately after receiving psilocybin, the placebo-first group had significant within-group reductions in depression and anxiety symptoms on five of six primary measures. These reductions persisted and were present at all three time points, including the final time point at 26 weeks after dose 2 (approximately 6.5 months). At follow-up, 6.5 months after the active psilocybin intervention, 60%−80% of participants had sustained their responder status on depression and anxiety scales (defined as a reduction ≥50% in score on the measure compared with baseline).
There is preliminary evidence that psilocybin may be efficacious in the treatment of substance use disorders. An open-label study by Johnson et al. (
30) enrolled participants who wanted to quit smoking (N=15) in a 15-week course of smoking cessation treatment coupled with psilocybin administration. The first 4 weeks of treatment consisted of cognitive-behavioral therapy, assigning a target quit date, and keeping a smoking diary. Psilocybin was administered at weeks 5 and 7, with an optional third psilocybin session at week 13. Participants were given a moderate dose of psilocybin (20 mg/70 kg) during the first experimental session and received a higher dose of psilocybin (30 mg/70 kg) at their second and third experimental sessions, unless they requested a moderate dose of psilocybin. The target quit date coincided with the first psilocybin session. During the sessions, research staff provided nondirective interpersonal support and did not deliver smoking cessation–specific content. Smoking abstinence was verified at all data collection points using exhaled carbon monoxide (CO level ≤6 ppm) and urinary cotinine measurements (level <200 ng/mL). At the 6-month follow-up, 12 of the 15 participants (80%) were laboratory-verified as abstinent; 10 participants (67%) remained abstinent at 12 months, and nine (75%) at 2.5 years. The pilot study has been extended to include 95 participants and should be completed by 2021 (ClinicalTrials.gov identifier 01943994).
Bogenschutz et al. (
31) evaluated open-label psilocybin for the treatment of individuals who met DSM-IV criteria for alcohol dependence and had at least two heavy drinking days in the previous 30 days (N=10). Participants also received psychotherapy, which included 14 sessions: seven sessions of motivational enhancement therapy, three preparation sessions, two psilocybin-assisted psychotherapy sessions, and two debriefing sessions. Participants received their first dose of psilocybin (0.3 mg/kg) after their first four psychotherapy sessions and their second dose (0.4 mg/kg) after their next four sessions, which was followed by four more psychotherapy sessions.
The primary outcome measures were the Stages of Change Readiness and Treatment Eagerness Scale, the Alcohol Abstinence Self-Efficacy Scale, the Penn Alcohol Craving Scale, and the Profile of Mood States. Two therapists were present throughout the psilocybin sessions, and their interactions with the participants were supportive and nondirective. Abstinence was not biologically verified and was based on self-report. The study found that abstinence significantly increased after the first psilocybin session at 4 weeks and was largely sustained through 36 weeks. Bogenschutz et al. are currently conducting a randomized clinical trial investigating the efficacy of psilocybin for treating alcohol dependence. The study is projected to enroll 180 participants and is expected to be completed in 2020 (ClinicalTrials.gov identifier 02061293).
Central to psilocybin-assisted therapy is the notion that participant response correlates with a psilocybin-induced “mystical” or “spiritual” experience. In the studies described above, the investigators noted correlations between symptom reduction and the participants’ appraisals of their psilocybin experiences as personally meaningful, as reflected by their scores on the 30-item Mystical Experience Questionnaire (MEQ-30) (
18,
30,
31). The MEQ-30 is a validated measure of mystical experience (
32) that assesses seven domains of mystical experiences: internal unity, external unity, noetic quality (feeling of perception or revelation during the experience), sacredness, positive mood, transcendence of time/space, and ineffability (difficulty of communicating or describing the experience to others) (
33). Confirmatory factor analyses have demonstrated the reliability and validity of the instrument, and external and convergent validity have been demonstrated by latent variable scores positively predicting psilocybin-related changes in attitudes, behavior, and well-being (
32).
Mystical experiences have many names—religious experiences, transcendental experiences, transforming moments, epiphanies—but are all characterized by personal transformations that lead to dramatic or “quantum” changes in a person’s sense of self and behavior (
34). In a prospective study, Griffiths et al. (
34) examined the long-term effects of a psilocybin-related mystical experience in individuals with no prior use of psilocybin when combined with meditation or spiritual practices. The total scores on the MEQ-30 and the Spiritual Experiences Scale both indicated healthy psychological functioning at 6-month follow-up, with the intensity of the psilocybin-induced mystical experience making the most significant contribution to the effect.
Although practitioners recognize that the acute presentation of a psilocybin-intoxicated individual closely resembles psychosis, hallucinogens such as psilocybin are not thought to precipitate a new psychotic illness but rather may unmask a psychotic disorder in those who are susceptible (
35,
36). In an analysis of 110 healthy study volunteers from 227 psilocybin administrations, researchers found no evidence of hallucinogen persisting perception disorder, prolonged psychosis, or other long-term impairment of functioning in any subjects (
37). Much of the research on the sequelae from psilocybin and other classic psychedelic use is from studies that screen participants for a history of psychiatric problems, regulate the dosage of the drug, and administer the drug in a controlled setting. These safeguards are intended to minimize the potential for adverse events.
Contrast this with the potential effects of psilocybin in an uncontrolled community setting. In an online survey (
38) of almost 2,000 people who answered positively to the question of whether, after taking psilocybin mushrooms, they “ever had a psychologically difficult or challenging experience (i.e., a bad trip)—that is, have you experienced significant fear, anxiety, or distress or anything else that you found psychologically difficult,” 39% of respondents reported that the experience was one of the most challenging experiences of their lifetime. Twenty-four percent of participants reported psychological symptoms lasting 1 week or longer (i.e., fear, anxiety, depression, or paranoia), 10% reported persistent symptoms for more than 1 year, and 7.6% sought professional help for psychological symptoms. Although this online survey is not rigorous enough to serve as a guide for clinical practice, it nevertheless points out potential concerns with the use of psychedelics in uncontrolled settings (
6).
In 2018, the FDA designated psilocybin a “breakthrough therapy” for treatment-resistant depression, giving it priority consideration in the regulatory process (
39). At this time, Compass Pathways, a London-based life sciences company, is starting phase 2B clinical trials in Europe and North America in 216 patients across 12–15 research sites for treatment-resistant depression, with additional phase 3 studies (
40–
42). The Usona Institute, a U.S. nonprofit medical research organization, is also planning phase 2 and 3 FDA-registration multisite trials to investigate psilocybin as a treatment for depression, anxiety, and mood disorders associated with end of life (
43). Two ongoing phase 2 randomized clinical trials are investigating psilocybin’s effects in patients with a diagnosis of obsessive-compulsive disorder to replicate and extend the initial findings of a study by Moreno et al. (
44) (published in 2006, outside the search date criteria for this review) (ClinicalTrials.gov identifiers 03300947 and 03356483). Additional studies are investigating psilocybin for the treatment of cocaine use disorder (ClinicalTrials.gov identifier 04052568), opioid use disorder (ClinicalTrials.gov identifier 04161066), anorexia nervosa (ClinicalTrials.gov identifier 04052568), and depression in early Alzheimer’s disease (ClinicalTrials.gov identifier 04123314).
Lysergic acid diethylamide (LSD).
LSD is an ergot derivative best known for its ability to induce powerful psychedelic, spiritual, and mystical experiences (
1,
45,
46). LSD has been described as a
psychoadjuvant or “nonspecific amplifier of the unconscious,” with effects that include weakening ego identification, accelerating and broadening thought processes and content, promoting novel thought associations, and modifying one’s interpretations and understanding of relationships and objects (
47–
49). It can induce feelings of closeness to others, enhance emotional empathy, enhance sociality, and acutely impair fear recognition (
50). At moderate to high doses, LSD enhances sensory perception, which can lead to illusions, dreamlike waking imagery, synesthesia, alterations in sound perception, and mystical experience (
48,
51–
53).
The hallucinogenic effects of LSD are thought to be mediated by several mechanisms: partial agonism at the 5-HT
2A receptor, binding to the 5-HT
1A, 5-HT
2C, and 5-HT
2B receptors (with affinity in descending order), and binding at dopamine D
2 receptors. It also causes glutamate release in the frontal cortex and increased functional connectivity and excitability in thalamic and cortical structures (
11,
54–
58). LSD does not interact with monoamine transporters and is more potently bound than all other tryptamines to the 5-HT
2A and 5-HT
1A receptors (
11). Other pharmacodynamic and pharmacokinetic mechanisms of LSD have been extensively explored (
59) but are outside the scope of this review.
Starting in the 1940s and continuing through the 1960s, there was a rise in the number of studies on potential uses of LSD in healthy volunteers as well as in treating psychiatric disorders (
16,
60). Observed psychological outcomes were initially thought to mimic schizophrenia, suggesting LSD as a potential model for psychosis (
1,
47,
61). Recent studies have shown that psychotic symptoms associated with LSD ingestion are more likely in healthy volunteers with premorbid schizoid and paranoid traits and persons with a family history of schizophrenia (
62). A large epidemiologic study of 130,000 adults in the United States did not find a link between psychedelic use (including LSD and psilocybin) and mental health problems or suicidal behavior (
63).
Studies have noted the experiential effects of LSD-induced behavioral changes in individuals with substance use disorders, and LSD has been recognized as a potential treatment for alcohol use disorder (
64). Several research groups have described LSD’s potential for symptom alleviation in individuals with mood disorders and in pain syndromes associated with end-of-life care (
16,
45,
65). Although preliminary LSD trials produced generally positive outcomes, clinical research on the therapeutic use of LSD was cut short in 1968, when the Drug Abuse Control Amendments were modified to make possession of LSD a misdemeanor and the sale of LSD a felony. LSD is currently classified as a Schedule I drug under the Controlled Substances Act (
66,
67).
Recently there have been a few small open-label studies outside the United States investigating LSD for the treatment of mood disorders, anxiety in the terminally ill, and migraine headaches (
16,
68). A group of Swiss and German researchers, Gasser et al. (
48), conducted a randomized controlled trial to examine the safety and efficacy of LSD-assisted psychedelic psychotherapy in patients with anxiety associated with medical disease (N=12), including malignancy, Parkinson’s disease, celiac disease, and ankylosing spondylitis. The primary outcome measure was the STAI trait and state forms completed at baseline, at 1 week, and at 2-month and 12-month follow-ups. At baseline, all participants scored >40 on the STAI state and trait, and half met DSM-IV criteria for generalized anxiety disorder. Participants were tapered off of antidepressant and antianxiety medications and received psychotherapy supplemented by two LSD-assisted psychedelic psychotherapy sessions spaced 2 to 3 weeks apart. Eight participants received a moderate dose of LSD (200 μg), and four participants received a low dose (20 μg), which was intended to act as an active placebo.
At the 2-month follow-up, mean trait anxiety did not significantly change in the high-dose LSD group compared with the placebo group, but mean state anxiety was significantly decreased in the high-dose LSD group compared with the low-dose (placebo) group. Comparing trait and state anxiety scores at baseline with those at the 2-month follow-up yielded effect sizes of 1.1 and 1.2, respectively. All four participants in the low-dose (placebo) group experienced increases in trait anxiety over time, and two of them also had increases in state anxiety (
69).
Swiss researchers Schmid and Liechti et al. (
69,
70) reported on short-term and long-term follow-ups after healthy volunteers (N=16) were given a single moderate dose of LSD (200 μg) as part of a randomized double-blind placebo-controlled crossover study with two experimental sessions. During the experimental sessions, participants rested in hospital beds and had the option of listening to music on headphones (no alternative entertainment was offered, and no specific guidance or therapy was provided). Participants were asked to complete the Persisting Effects Questionnaire (
71), the Mysticism Scale, lifetime version, the Death Transcendence Scale, and the NEO Five-Factor Inventory at study screening and again 1 month and 12 months after their LSD session.
One and 12 months after LSD administration, the Persisting Effects Questionnaire showed significant increases in positive attitudes about life or self, positive mood changes, altruistic/positive social effects, positive behavioral changes, and well-being/life satisfaction that participants attributed to their LSD experience. The Mysticism Scale total score was increased, with significant increases in introvertive and extrovertive factor scores. The Death Transcendence Scale total score and mysticism subscale scores were also significantly increased at 1 and 12 months, and the NEO Five-Factor Inventory ratings of conscientiousness were significantly higher at 12 months. After 12 months, 10 of 14 participants (71%) rated their LSD experience “among the 10 most meaningful experiences” in their lives, and five participants rated it “among the five most spiritually meaningful experiences” in their lives. This study suggested positive effects of LSD on attitudes, mood, and behavior, which may have implications for the treatment of psychiatric disorders (
70).
Neuroimaging researchers Mueller et al. (
72) conducted a double-blind placebo-controlled randomized crossover study investigating the effects of LSD (100 μg) on amygdalar activity during processing of fearful stimuli in healthy subjects (N=20). At the point of anticipated peak effect, 2.5 hours after LSD ingestion, participants underwent fMRI scans while viewing images of faces depicting various degrees of fear, anger, happiness, or neutral expressions taken from the Ekman and Friesen series of Pictures of Facial Affect. All participants were crossed over to the other condition and scanned with the same protocol. Compared with placebo, LSD produced a significant decrease in left amygdalar reactivity to fearful stimuli and impaired recognition of fearful faces, but it did not affect recognition of neutral, happy, or angry faces. It was also noted that LSD administration was associated with decreased activity in the right medial prefrontal cortex compared with placebo. The investigators interpreted the results as indicating that LSD may modify the processing of biases toward negative stimuli, which play a role in depression and anxiety disorders. They also suggested that LSD might be useful for reducing perceptions of negative emotions, ameliorating social cognitive deficits, and facilitating therapeutic alliance.
Recently, there has been emerging interest in microdosing LSD, the practice of taking doses below the perceptual threshold at 3- to 5-day intervals in an effort to trigger a cellular response. Mainstream media publications and subjective reports have suggested that microdosing LSD at 10–20 μg might induce positive effects, such as promoting creativity and enhancing mood, without the full experience of psychedelic effects (
73,
74). Currently, there is no available scientific evidence to support the practice of microdosing. In fact, LSD doses of 13 and 26 μg (N=20) have been shown to produce measurable subjective and physiological effects with minimal effects on cognition and creativity (
75). It is worth highlighting that low-dose LSD (20 μg) received by the active placebo group in the Gasser et al. study mentioned (
48) above was associated with worsening anxiety in people with comorbid medical illness. While this finding may be attributable to resampling over time or placebo nonexpectancy, it may also be ascribed to microdosing. The Beckley Foundation intends to study the neurobiological and clinical effects of LSD microdosing as a strategy for cognitive enhancement in an upcoming investigation, but specific details were unavailable at the time of writing.
While the current LSD clinical research is limited, there are several new clinical investigations on the horizon in Switzerland. These studies will examine LSD as a treatment for patients suffering from anxiety with or without a life-threatening disease (ClinicalTrials.gov identifier 03153579), LSD-assisted psychotherapy for patients with illness-related anxiety (ClinicalTrials.gov identifier 00920387), and LSD-induced altered states of consciousness (ClinicalTrials.gov identifier 03321136).
Ayahuasca.
Ayahuasca is a decoction prepared through the combination of
Banisteriopsis caapi and
Psychotria viridis, two plants native to the Amazon basin (
76–
79). Ingested orally, the mixture is known to induce effects by actions of β-carboline alkaloids (namely, harmine derivatives) found in
Banisteriopsis caapi and
N,N-dimethyltryptamine (DMT) in
Psychotria viridis (
76,
78). The preparation works synergistically, in that β-carboline alkaloids inhibit monoamine oxidase A (MAO-A) (
80), preventing peripheral degradation of DMT, a serotonin transporter and norepinephrine transporter inhibitor as well as releaser of 5-HT and agonist at 5-HT
1A, 5-HT
2A, 5-HT
2C, and 5-HT
2B receptors (with affinity in descending order) (
11,
80,
81). The environment in which the substance is ingested, the user’s expectations, and pharmacodynamic interactions of the decoction’s components are all thought to influence outcomes associated with ayahuasca ingestion (
77).
Ayahuasca is associated with a wide range of subjective effects, including auditory and visual hallucinations, altered sensorium, altered spatial perceptions, and euphoria (
77,
82), as well as mystical and noetic experiences (
77). Psychotic episodes have been documented in association with ayahuasca intoxication, usually in persons with a personal or family history of mood disorders, psychotic disorders, or substance use disorders (
36,
60,
83). These ayahuasca-induced psychoses are not generally prolonged. It has been documented that psychoses can be mitigated by screening individuals for preexisting psychiatric disorders, but conclusions regarding the relationship between ayahuasca and prolonged psychotic episodes are drawn from small sample sizes, therefore limiting generalizability (
60,
84).
Ayahuasca consumption has been associated with traditional practices among indigenous groups of the northwestern Amazon region, but the past several decades have seen a growing international interest in its possible therapeutic effects (
77,
85). The U.S. Supreme Court has sanctioned the use of ayahuasca for religious and spiritual practices (
86) by groups such as União do Vegetal and Santo Daime, but clinical trials in the United States remain nonexistent because DMT, a component of ayahuasca, is a Schedule I controlled substance.
Clinical investigations with ayahuasca outside the United States have begun in the past several years. Brazilian researchers Osório et al. (
87) conducted a small (N=6) open-label clinical trial investigating the efficacy of ayahuasca in patients with depression who had not responded to at least one trial of an antidepressant medication. All patients met criteria for major depressive disorder based on the Structured Clinical Interview for DSM-IV and were admitted to a psychiatric unit for 2 weeks for drug washout prior to ayahuasca administration. The HAM-D and Montgomery-Åsberg Rating Scale (MADRS) were administered 10 minutes before ayahuasca administration and again 40, 80, 140, and 180 minutes afterward, with follow-up assessments 1, 7, 14, and 21 days later. Participants drank a standard dose (2.2 mL/kg) of ayahuasca (containing 0.8 mg/mL DMT, 0.21 mg/mL harmine, and no harmaline as measured by gas chromatography/mass spectrometry) prepared by the Santo Daime community. All participants were discharged from the psychiatric unit 24 hours after ayahuasca administration. Mean HAM-D score was reduced by 62% 1 day after drug administration (p=0.01), with an even more pronounced reduction of 72% (p=0.01) 7 days after drug administration. The mean MADRS score was reduced by 82% at 7 days (p=0.009), with a sustained effect at 21 days. Investigators noted that the most significant antidepressant effects were observed for expressed sadness, pessimistic thinking, suicidal ideation, and difficulty concentrating.
Given the positive therapeutic signal of their pilot study, the same research team conducted a replication study with a larger sample (N=17) (
88). The mean baseline HAM-D score for this group was 19.4, and the mean baseline MADRS score was 25.6. Symptoms, as measured by both scales, significantly decreased acutely, starting 80 minutes after drug administration. At 21-day follow-up, the mean HAM-D score was 7.56, representing a highly statistically significant mean change of −11.4 points (p<0.0005). Positive findings in the earlier study were replicated, but because neither study was randomized, double-blinded, or placebo-controlled, the results must be viewed as preliminary. Although vomiting occurred in about half the participants, participants generally described the ayahuasca session as a pleasant experience, and no serious adverse events were observed in either study.
Currently, the data are insufficient to support the use of ayahuasca in the clinical setting. The clinical research involving ayahuasca, which includes promising preliminary results for the treatment of depression, is limited by several factors, including lack of chemical analyses to confirm the exact ingredients in the ayahuasca drink used in the studies. A multitude of additional compounds have been described across indigenous preparations, including, among others, caffeine, nicotine, cocaine, and scopolamine (
78). In assessing the aforementioned studies, one must be cognizant of the fact that ayahuasca was administered as a nonstandardized concoction. Randomized clinical trials using pharmacologically pure compounds are necessary to advance our knowledge about the therapeutic potential of ayahuasca.
3,4-Methylenedioxymethamphetamine (MDMA).
MDMA is a ring-substituted phenethylamine with structural similarities to amphetamine and mescaline. MDMA was synthesized by Merck & Co. in 1912 as a potential therapeutic agent to decrease clotting time and to prevent hemorrhaging (
89). The compound did not prove efficacious for use as a hemostatic drug, but its psychotropic properties were recognized. Chemist Alexander Shulgin resynthesized MDMA in 1976, and the first published report characterizing the psychoactive effects of MDMA appeared in 1978 (
90).
Despite the lack of systematic research into its efficacy and safety, some psychotherapists began using MDMA to improve the outcome of psychotherapy sessions with the goal of enhancing their patients’ insights and understanding of their psychological problems. MDMA was associated with feelings of emotional well-being and was described as “penicillin for the soul” (
90).
These psychoactive properties encouraged MDMA’s use as a recreational drug. In the early to mid-1980s, MDMA was illicitly synthesized and distributed under the street name “Ecstasy” and became popular for facilitating an altered emotional state at dance parties called “raves.” Because of concerns about abuse liability and neurotoxicity, the DEA emergently classified MDMA as a temporary Schedule I substance in 1985, and then permanently classified it as such in 1988.
MDMA and other
3,4-methylenedioxy- substituted phenethylamines have been postulated to represent a new class of pharmacological agents, termed entactogens, with effects only partially overlapping those of psychostimulants and serotonergic hallucinogens (
91–
93). The effects of MDMA are believed to be mediated by a number of mechanisms, including monoamine release, serotonin and norepinephrine transporter reuptake inhibition, monoamine oxidase inhibition, partial agonism of serotonin receptors (5-HT
2A, 5-HT
1A, and 5-HT
2C receptors), and increase in blood concentrations of oxytocin (
94–
98). To date, studies with healthy volunteers have confirmed that MDMA produces an easily controlled and reversible state of altered consciousness characterized by euphoria, empathy, well-being, insightfulness, extraversion, positive mood, gregariousness, feelings of authenticity, increased access to emotionally intense material, increased interpersonal trust, and compassion for oneself and others (
96,
99–
103). In the clinical population, anxiety has been reported in a majority of study participants, and painful emotions such as grief, fear, and rage are not uncommon in participants with a diagnosis of PTSD (
104–
106).
The first double-blind placebo-controlled MDMA study in the United States was conducted in 1994 (
107) and was followed up by two additional phase 1 trials (
91,
108). A single dose of MDMA causes transient but tolerable increases in heart rate, blood pressure, and body temperature in healthy subjects (
109). Subsequent placebo-controlled studies in Europe confirmed these general safety and tolerability findings and demonstrated that the processing of contextual information is left intact after MDMA ingestion (
110,
111).
A double-blind fMRI randomized clinical trial in healthy volunteers (N=9) (
112) showed that during peak drug effect, MDMA decreased amygdalar reactivity in response to angry faces but not fearful faces and enhanced ventral striatum activity in response to happy faces from the Ekman and Friesen series of Pictures of Facial Affect. Volunteers receiving MDMA were also better able to verify positive facial expressions and found it more difficult to identify negative ones, compared with volunteers who received placebo. These findings of reduced response to threat and enhanced responses to reward provided important insights into MDMA’s effects on emotional information processing (
112,
113).
In 2010, Mithoefer et al. (
106) completed the first phase 2 randomized controlled trial investigating the efficacy of MDMA in treating chronic PTSD (N=23). The study enrolled adults with a DSM-IV-TR diagnosis of chronic PTSD. Inclusion criteria also included treatment-resistant symptoms (defined as a score ≥50 on the Clinician-Administered PTSD Scale [CAPS]) and previous failure of at least 3 months of an SSRI or selective serotonin-norepinephrine reuptake inhibitor in addition to 6 months of psychotherapy (the specific type of psychotherapy was not specified). Study participants received two experimental sessions of either manualized MDMA-assisted psychotherapy with active drug (125 mg orally with an optional supplemental dose of 62.5 mg) (N=12) or placebo (N=8). The manualized therapy was developed for the study based on principles of Holotropic Breathwork (
114) and LSD psychotherapy (
115), and it emphasized a nondirective supportive approach (
104,
105).
The primary outcome measure was mean change in CAPS total scores measured at baseline, 4 days after each experimental session, and 2 months after the second experimental session. Baseline mean CAPS scores were 79.6 (SD=8.1) for the placebo group and 79.2 (SD=6.6) for the MDMA group (p=0.966). Three to 5 days after the first experimental session, the participants’ CAPS scores were 74.1 (SD=10.3) for the placebo group and 37.8 (SD=8.4) for the MDMA group (p=0.013). Three to 5 days after the second experimental session, CAPS scores were 66.8 (SD=8.0) for the placebo group and 29.3 (SD=6.5) for the MDMA group (p=0.002). Two months after the second experimental session, CAPS scores were 59.1 (SD=9.4) for the placebo group and 25.5 (SD=7.7) for the MDMA group (p=0.013). A significantly greater proportion of the MDMA group (10 of 12, 83.3%) than the placebo group (2 of 8, 25%) met criteria for categorical response (reduction ≥30% from baseline in CAPS score). All placebo-treated participants were offered the option of subsequent open-label crossover. Seven of eight chose to cross over, and all seven had a clinical response 4–6 weeks after two MDMA sessions. The mean change in CAPS score in this group (N=7) was −31.7 (SD=15) (p<0.05).
CAPS scores obtained 17–74 months after the two MDMA-assisted psychotherapy sessions were examined in a prospective long-term follow-up study (
116). Sixteen participants completed all measures over 3.5 years (duration of follow-up: mean=45.4 months, SD=17.3). Among completers, no significant change was observed in mean CAPS scores from the point of exit from the trial (mean=24.6, SD=18.6) to the final follow-up assessment (mean=23.7, SD=22.8). On average, the group maintained statistically and clinically significant PTSD symptom relief, suggesting a potential for durable therapeutic effect from MDMA-assisted psychotherapy.
Most recently, Mithoefer et al. (
105) completed a three-dose phase 2 double-blind randomized controlled trial investigating the efficacy and dose-response relationship of MDMA-assisted psychotherapy for the treatment of chronic PTSD in service personnel, firefighters, police officers, and veterans (N=26). All participants had a diagnosis of PTSD for at least 6 months, had a baseline CAPS total score ≥50, and had failed to respond to, or tolerate, previous pharmacotherapy or psychotherapy trials. Participants were required to taper and remain off of psychotropic medications during study participation. Participants were randomly assigned to receive MDMA at a low dose (30 mg; N=7), a moderate dose (75 mg; N=7), or a high dose (125 mg; N=12) in two blinded psychotherapy sessions spaced 1 month apart. In all of the MDMA sessions, participants had the option of receiving a supplemental dose of half of the initial dose 1.5–2 hours after the initial dose. During the MDMA sessions, two therapists, a male and female co-therapy team, performed manualized MDMA psychotherapy (the same nondirective supportive therapy approach used in the pilot study described above). The primary outcome measure was the mean change in CAPS score from baseline to 1 month after the second experimental MDMA session. The moderate- and high-dose groups had significantly greater reductions in PTSD symptom severity from baseline than the low-dose group (low-dose group: −11.4, SD=12.7; moderate-dose group: −58.3, SD=9.8; p=0.0005; high-dose group: −44.3, SD=28.7; p=0.004). No significant differences were found between the moderate- and high-dose groups (p=0.185). Remission was achieved in six of the seven participants (86%) in the moderate-dose group and seven of the 12 participants (58%) in the high-dose group, compared with two of the seven participants (29%) in the low-dose group. Additionally, compared with the low-dose group, more participants in the moderate- and high-dose groups met criteria for clinical response (defined as a reduction >30% from baseline in CAPS score): 29% in the low-dose group, 100% in the moderate-dose group, and 67% in the high-dose group.
In 2016, the FDA approved the MAPS investigators’ design for two phase 3 clinical trials investigating MDMA for the treatment of PTSD (
117). In 2017, the FDA designated MDMA as a “breakthrough therapy” based on its use in assisting psychotherapy for the treatment of PTSD, giving it priority consideration in the regulatory process (
118).
Additional trials investigating the efficacy of MDMA for social anxiety disorder in adults with autism spectrum disorder (ClinicalTrials.gov identifier 02008396) and for anxiety associated with a life-threatening illness (ClinicalTrials.gov identifier 02427568) have been completed but are outside the scope of this review.