Introduction
Cervical vagus nerve stimulation (VNS) involves direct implantation of stimulating electrodes, wires, and a pulse generator, which electrically stimulates the left cervical bundle of the vagus nerve, Cranial Nerve X (CN X). Cervical VNS is a relatively safe and moderately effective therapy for treating intractable epilepsy or treatment resistant depression [
1,
2]. Unfortunately, risks involved in surgical implantation, as well as high procedural cost ($30,000—50,000) makes it less appealing, and limits access. This is especially so if insurance companies do not reimburse the procedure (as is currently the case regarding VNS to treat depression). Additionally, only about 30% of implanted patients demonstrate a clinical response, despite undergoing the procedure. A non-invasive method to stimulate the vagus as an alternative treatment, or to determine ultimate VNS responders before they have cervical surgery would likely improve VNS use as a treatment modality.
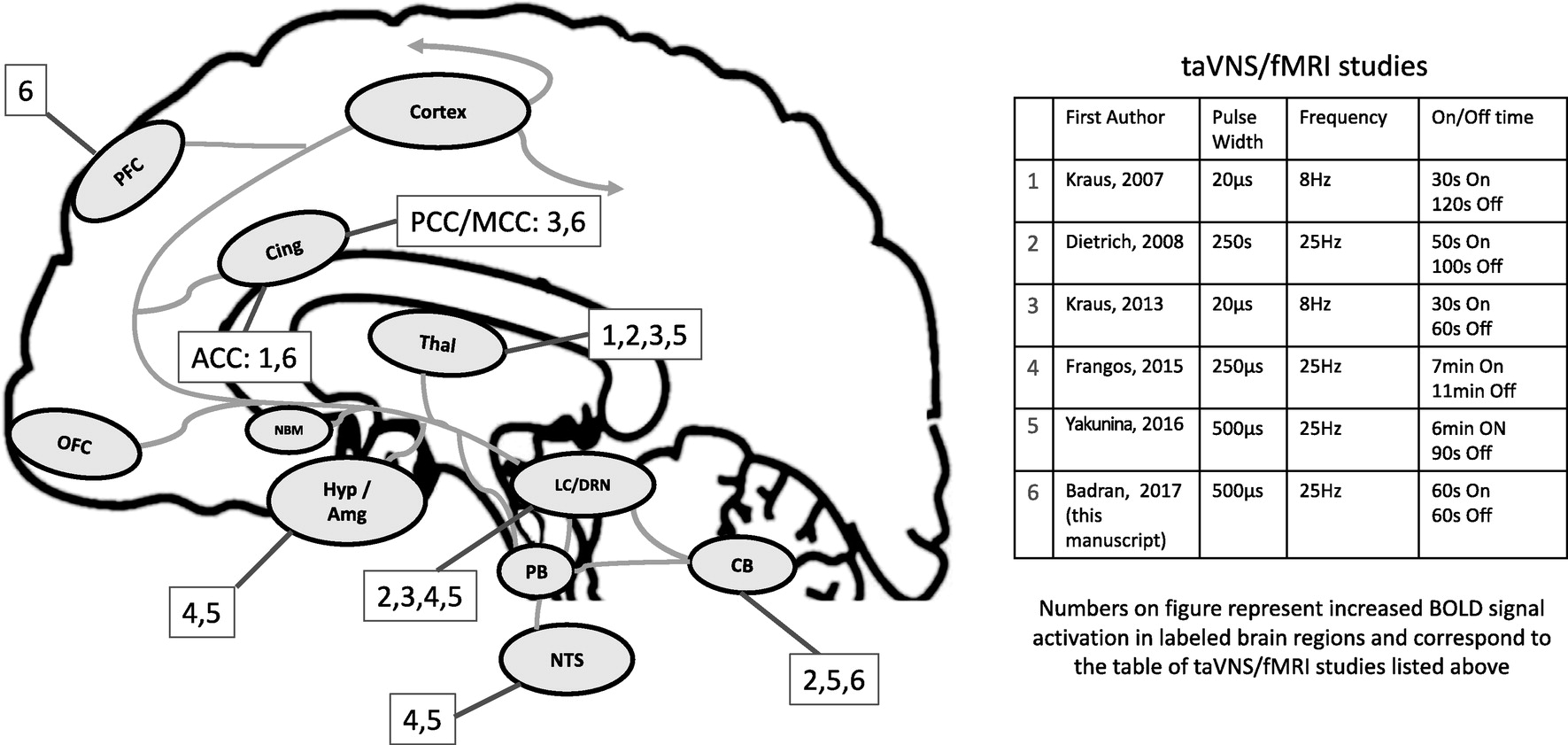
The numerous behavioral benefits of cervical VNS likely arise from its highly diffuse afferent brain targets. The vagus nerve enters the central nervous system in the nucleus tractus solitarius (NTS) [
3,
4] (
Fig. 1). From the NTS, there are direct afferent projections to the parabrachial complex (PB); from here, projections are sent to the locus coeruleus (LC) and raphe nuclei [
5]. Further ascending projections from the PB ascend to higher brain regions [
6]. Interestingly, Krahl et al. lesioned the LC and caused the anti-epileptic effect of VNS to disappear [
7], demonstrating the role of the norepinephrine (NE) released by the LC as central to the mechanism of anti-epileptic VNS. NE is not the only neurotransmitter involved in the afferent vagal pathway. Serotonin (5-HT) is released through direct projections from the dorsal raphe nucleus [
8], the primary source of serotonin, which independently has a wide range of ascending brain targets (thalamus, cerebellum, hypothalamus, amygdala, insula, cingulate and frontal cortex), many of which are also targeted by the ascending LC pathway (
Fig. 1).
Aside from NE and 5-HT, which are often described as the key neurotransmitters involved in VNS, a dopaminergic basis of response has been suggested. A recent study by Conway and colleagues used
17Fluorodeoxyglucose positron emission tomography (PET) imaging in humans to reveal VNS responder-specific increased activation of the ventral tegmental area (VTA), one of two regions responsible for dopamine (DA) release [
9]. Non-responders were not shown to have this increase, suggesting DA may be an alternative VNS mechanism. Only DA metabolites (not NE or 5-HT) were also reported to be increased in cerebrospinal fluid (CSF) after VNS therapy [
10].
The auricular branch of the vagus nerve (ABVN) spans from the main bundle of the vagus nerve and innervates the human ear, although its afferent projections are still not well understood. The ABVN is the target of a novel form of noninvasive brain stimulation, known as transcutaneous auricular vagus nerve stimulation (taVNS). Modern taVNS was perhaps first described by Ventureyra in 2000 [
11], with several more recent studies using this novel form of neuromodulation [
12–
17], including 5 preliminary fMRI trials [
18–
22]. Importantly, these taVNS/fMRI trials have widely different methodology with varied results. For example, the studies employed a range of stimulation targets, parameters, durations, and control site. The field of taVNS still lacks a consensus on which parameters and ear targets are the most biologically active, and whether the brain regions secondarily activated depend on specific parameters. That is, can one sculpt the central brain activation of taVNS by simply varying target and dose parameters? To better visualize this, we have overlaid the various prior studies' parameters and brain activations on
Fig. 1.
Currently, there is a debate as to which anatomical target is most biologically active. There has been only one human auricle dissection study to date conducted in 7 German cadavers [
23] that demonstrates the cymba conchae and tragus as the highest density of ABVN projections. Groups have conducted studies stimulating both tragus and cymba conchae in fMRI paradigms, with both sites delivering promising results. Without intricate microdissection and combined stimulation studies, along with individual anatomical differences, it is difficult to determine which site truly activates the main bundle of the vagus. It is also important to consider stimulation parameters in VNS paradigms, as Mu et al. demonstrated that increasing pulse width of cervically implanted VNS increases BOLD signal activation in the vagal network [
24]. Similar to cervical VNS, taVNS likely varies as a function of stimulation characteristics and thus should be assessed parametrically.
Our group has conducted a series of safety and parametric physiological work demonstrating the autonomic effects of electrical stimulation of the tragus. These findings suggest the tragus is a likely, biologically active target [
25]. We proceeded to develop a taVNS system that is compatible with functional magnetic resonance imaging (fMRI) in order to further test the afferent brain activation of electrical stimulation of the tragus. Using this novel technique and the parameters that produced heart rate changes, we conducted a concurrent taVNS/fMRI imaging study to determine the afferent pathway of left tragus stimulation in healthy adults.
Methods
Overview
We conducted a two-visit, single blind, crossover fMRI trial exploring the effects of active taVNS stimulation compared to control earlobe stimulation. Participants had two identical scanning visits, separated by at least one day to avoid carryover effects. Scans were acquired at the MUSC Center for Biomedical Imaging research dedicated 3T Siemens Trio location. This study was approved by the MUSC Institutional Review Board (IRB) and is registered on ClinicalTrials.org (NCT02835885). All participants signed written informed consent prior to scanning.
Participants and Inclusion Criteria
17 healthy individuals (8 women) were enrolled after meeting the following inclusion criteria: Age 18–45, no personal or family history of seizure, mood, or cardiovascular disorders, no facial or ear pain, no recent ear trauma, no metal implants including pacemakers, not pregnant, no dependence on alcohol or recent illicit drug use, not taking any pharmacological agents known to increase seizure risk (e.g., bupropion, neuroleptics, albuterol, theophylline, antidepressants, thyroid medications, or stimulants) and MRI contraindications (e.g., metal in body, pregnancy, and claustrophobia).
fMRI Scanning
All MRI scanning was conducted using a Siemens Trio TIM 3.0T system and a 32-Channel head coil. Individuals were positioned head-first supine in the MRI scanner, and foam pads were used to stabilize their heads and to minimize movement.
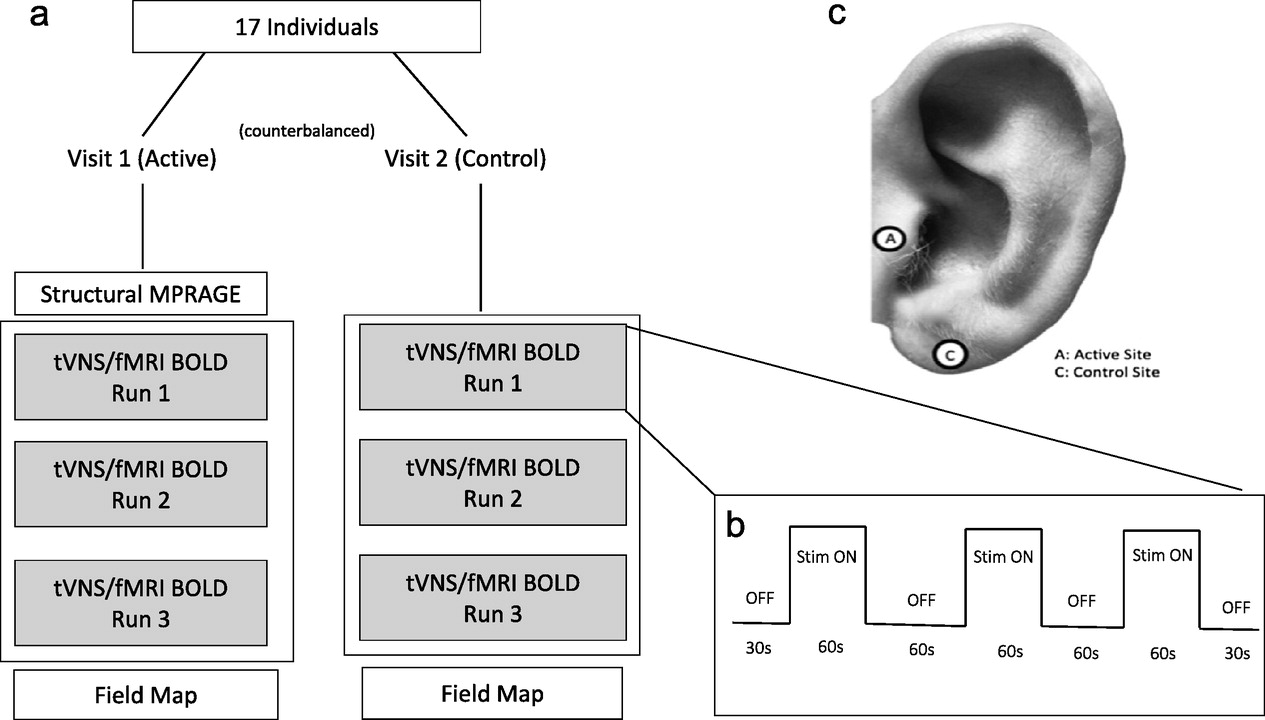
Each of the two scanning sessions lasted approximately 30 min, during which multiple scanning sequences were acquired (
Fig. 2a). First, for the purpose of warping each participant into a standard space template, a high resolution anatomical MPRAGE (TR: 1900 ms; TE: 2.26 ms; Voxel size: 1 mm
3; 208 slices, FA: 9 deg) was collected. Following the anatomical image, three identical taVNS/fMRI scans were collected, during which participants received either active or control concurrent taVNS. Lastly, a field map was acquired to correct for distortions due to magnetic field inhomogeneity.
The concurrent taVNS/fMRI scans were conducted using echo- planar imaging (EPI) sequence (TR: 2800 ms; TE: 35 ms; Voxel size: 3.0 mm
3; 47 slices, FA: 76 deg) in a block design described in
Fig. 2b. Each scan run was identical, consisting of three 60s "ON” periods, each separated by 60s "OFF” periods. A 30s "OFF” period also preceded the first and proceeded the last "ON” periods. Scans totaled 6 min each. The stimulation was synchronized with the start of each taVNS/fMRI BOLD sequence acquisition (from 0:00 and ran to 6:00 for each taVNS/fMRI run), and was triggered upon first fMRI volume acquisition using an automated stimulation system delivering TTL pulses to the constant current stimulator at a specific frequency and duration. Timing validation was confirmed with the console timer after each individual stimulation session. Upon completion of each taVNS/fMRI scan, individuals were asked via intercom how many stimulation blocks they sensed in order to verify signal transmission into the scanner and all three "ON” blocks were delivered. Participants were also asked to rate their pain on a visual response scale (NRS) from one (no pain) to ten (extreme pain).
Concurrent taVNS/fMRI System
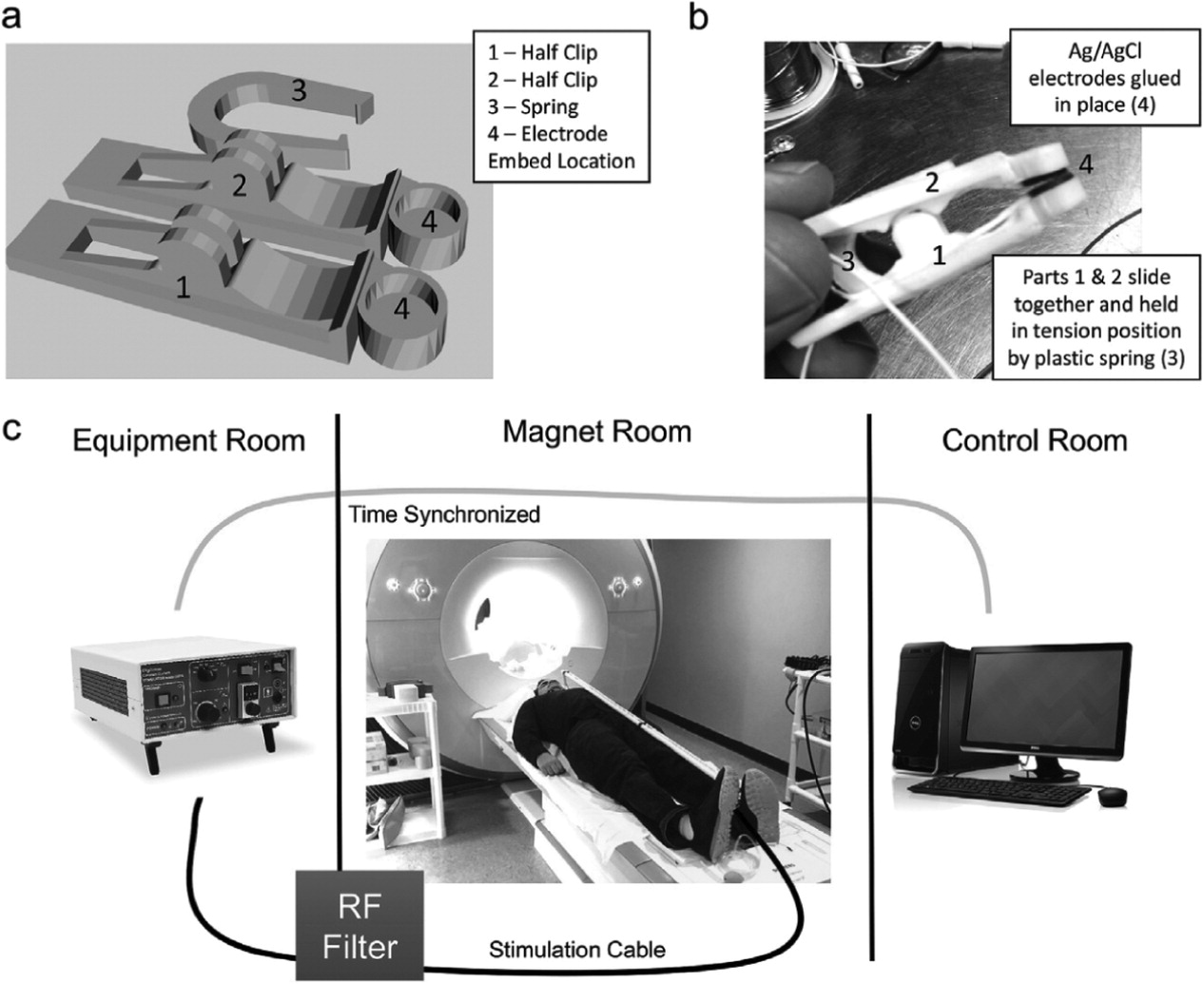
Stimulation was delivered via custom developed stimulating electrodes pictured in
Fig. 3a+b. The design of the electrodes was based on a freely available clip design (Clip by J_Hodgie, creative commons non-commercial attribution,
www.thingiverse.com/thing:5580) and modified in order to fit round metal electrodes at the clip ends. Computer assisted drawings of the electrode clamps were generated in SketchUp (Timble Navigation, USA) and subsequently 3D-printed in ABS plastic at the MUSC Brain Stimulation Laboratory. The round, unipolar stimulation electrodes were 1 cm in diameter, made of Ag/AgCl, and affixed to the 3D-printed clamps using cyanoacrylate. Copper was used for all wiring. Ten20 conductive paste was used as a conductor for the electrodes.
Constant current stimulation was delivered using a Digitimer DS7a set to <400 V. Lead wires were attached to the Digitimer output and connected to a radio frequency (RF) patch panel in the wall between the equipment room and magnet room using a serial connector on both sides.
Fig. 3c shows the taVNS/fMRI setup. Wire was run from the patch panel in the magnet room towards the foot of the MRI scanner, where it was then run on top of the participant who was laying supine head first on the scanning table. 1/2-inch PVC piping was used to insulate the wires, and rested on the participant's abdomen. Stimulation electrodes were clamped to the individual's tragus or earlobe, depending on active or control condition.
taVNS Parameters and Stimulation Targets
The parameters used for this fMRI trial were 500 μs 25 Hz (monophasic square waves) based on previous autonomic effects (manuscript under review and attached for reviewers). Perceptual threshold (PT) was determined while the participant was laying in the scanner with electrodes attached. Stimulation current was later set to 200% of PT. Perception of stimulation was relayed to the equipment room via intercom, and the current was calibrated to the minimum perceptual level for each participant. Active stimulation was delivered to the left tragus, control stimulation to the left earlobe (
Fig. 2c).
Data Processing and Analysis
All images were converted from DICOM to NifTI using dcm2nii applet. All further processing and analysis were performed in SPM 12 software (UCL) using MATLAB R2012a (The MathWorks Inc, Natick, MA). First, normalization parameters were derived from the segmentation of the whole brain anatomical images. Skull stripped anatomical images were created to improve functional to anatomical coregistration. Next, the functional images were realigned to reduce motion related variance. The mean image from realignment was coregistered to the skull stripped anatomical image using a normalized mutual information algorithm. The estimated coregistration parameters were combined with the normalization parameters in a single step and applied to the functional data. Finally, the data was smoothed using an 8 mm FWHM Gaussian smoothing kernel. No participants were excluded on the basis of the estimated movement parameters.
Three stimulation “ON” periods were modeled (onset times: 30s, 150s, 270s; duration 60s) in a block design. At the subject level, “ON” blocks were convolved with the canonical hemodynamic response function provided by SPM12. Contrast maps of “ON” stimulation were carried forward into a second level model, for group-level analysis of condition effects. Single sample t-tests of all individual stimulation blocks were considered in relation to the implicit baseline (active: ON > OFF, ON < OFF; control: ON > OFF, ON < OFF). An overall taVNS paired t-test contrast was also conducted (active ON > OFF > control ON > OFF; active ON<OFF > control ON < OFF). Lastly, a mask was created to explore the brain stem regional activations on all of the second level analyses.
Discussion
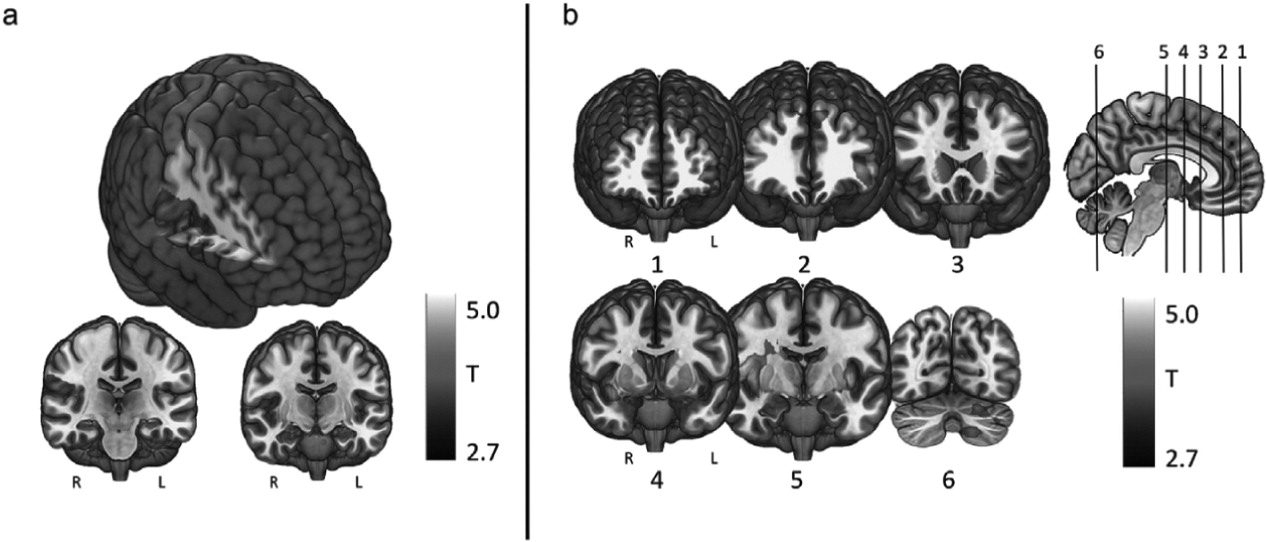
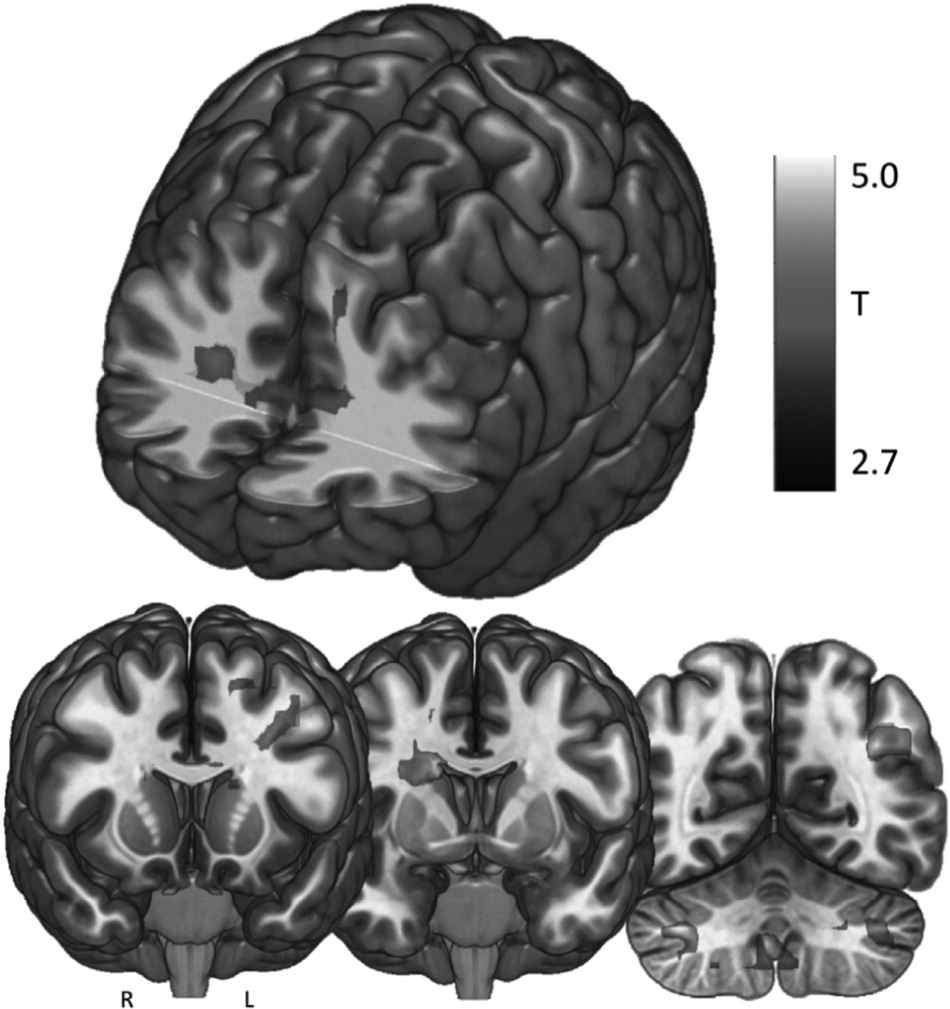
We investigated the direct brain effects of taVNS to either the left tragus (active) or earlobe (control) using a novel taVNS/fMRI paradigm in 17 healthy individuals. Each participant attended two scanning sessions, in which both left tragus (active) and left earlobe (control) stimulation was administered in order to determine the afferent brain effects of electrical stimulation of the ABVN. Our findings reveal that active taVNS in healthy young adults at 500 μs 25 Hz delivered for 60 s repeated 3 times over 6 min produces significant BOLD activations throughout cortical, subcortical, and cerebellar brain regions associated with the afferent vagal pathway. In contrast, control stimulation of the earlobe exclusively produces a contralateral somatosensory BOLD signal response in the postcentral gyrus representation of the face. When control response is subtracted from the active response in the overall contrast of active > control, significant activations emerge throughout the cingulate gyrus (bilateral ACC, bilateral mid-cingulate), frontal cortex (left middle and frontal gyrus), cerebellum, and right caudate. These activations are presumably due to stimulation of vagus afferents arising from the ear.
Within this trial, we describe two effects: 1) the somatosensory cortical representation of the ear, and 2) the cortical and subcortical direct brain effects of stimulating the ABVN. Penfield described the homuncular representation of the human primary sensory cortex [
28], and notably the ear is omitted from these trials. To date only been two studies have explored ear somatosensory representation [
29,
30], the first using magnetoencephalography (MEG) and the second using fMRI. Similar to the results in the current study, both describe the somatosensory response of the left ear being represented on the contralateral somatosensory cortex in the face and neck areas. The MEG findings demonstrated that somatosensory evoked magnetic fields (SEFs) were produced in response to slow (1 Hz), ultra brief (0.05 ms pulse width) electrical stimulation of the earlobe. The follow-up MRI findings by the same group confirm initial MEG findings that slow (2 Hz), brief (0.5 ms pulse width) electrical stimulation solely activates contralateral postcentral gyrus. Although our stimulation current was faster (25 Hz compared to 1 and 2 Hz), the pulse width was identical and our control stimulation findings are similar to these two sequential trials conducted by Nihashi et al.
The afferent pathway of the ABVN is still poorly understood, although we hypothesize that it activates the main vagal afferent pathway (via the NTS, LC, and upstream cortical projections) [
7,
31–
33]. To date, including this trial, there have been six taVNS/fMRI studies exploring the direct brain effects of electrical stimulation to the ear (
Table 3). Findings vary widely, as do the methods. As demonstrated in prior cervical VNS/fMRI trials, parameters have a direct effect on BOLD response [
34]. However, two of the prior taVNS trials explored 250 μs pulse width stimulation [
20,
21] and two others administered 20 μs stimulation [
18,
19]. Our trial used a 500 μs 25 Hz parameter similar to Yakunina et al. [
22], with similar findings. Like the Yakunina group, we demonstrate that tragus stimulation produces significant increased activation in the angular gyrus, caudate, cerebellum, cingulate, and frontal cortex. These regions in general are also found to be activated throughout the other shorter pulse width trials listed in
Table 3 and are afferent targets of the vagus nerve pathway, suggesting that ABVN stimulation enters the vagal bundle and projects to the brain via the brainstem.
Another major difference between these trials was the duration of stimulation, or "ON” period, during scanning. Three studies stimulated for less than one minute [
18,
19,
21], while the most recent trials stimulated for six and seven minutes, respectively [
20,
22]. In our trial, stimulation was delivered for one-minute blocks. The studies utilizing long stimulation periods reported BOLD signal activations in the brainstem region, while the prior three trials did not. Even in ideal conditions, breathing, heart rate, subject motion, and swallowing artifacts make imaging the small brainstem regions such as the LC and NTS a challenge. It is plausible that we did not discover any brainstem activations due to our short stimulation period of one minute, or due to our whole brain imaging approach (larger slices for wider brain coverage). Future studies should consider longer stimulation periods and scans optimized for imaging with rapid, thin slice acquisition of those regions, rather than whole brain scans if imaging of brainstem nuclei is a goal.
Interestingly, of all prior studies, ours is the only one that reflects the bilateral activation of the ACC and left dorsolateral prefrontal cortex (DLPFC) via taVNS. This may reveal a potential mechanism for the anti-depressant effect of cervically implanted VNS as well as the proposed anti-depressant effect of taVNS that has been described in the literature [
17,
35,
36]. The ACC is involved in cognition and emotional processing [
37,
38] and may play a key role in the depressions, expressing reduced glutamate release [
39–
41] and reduced glial cell density [
42–
44] in pathologic conditions. The ACC also has been used as a longitudinal predictor of treatment response in depression [
45,
46]. The left DLPFC has been demonstrated to be hypoactive in depression [
47,
48] and is a target of high frequency rTMS for its treatment [
49–
52]. Strong bilateral insular activity in the active contracts compared to control stimulation may also be of importance, as the insula has been implicated as a key region in models of biomarkers for mood disorders [
53,
54]. Presented with significant BOLD activations in regions of the brain associated with major depressive disorder (MDD), it is reasonable to consider taVNS as approach to treating MDD.
This trial establishes the left tragus as a biologically active ear stimulation site, allowing for transcutaneous stimulation of the ABVN and its afferent vagal network. The parameters used in this study (500 μs, 25 Hz, 60s ON, 60s OFF) are revealed to significantly activate these networks and produce reliable activation of vagal afferent networks. These findings are similar to the Yakunina group findings [
22], which is the only prior study that employed the 500 μs 25 Hz parameter. Our suggested duty cycle (1min on/1min off) is tolerable and can be easily translated into a disease population.
Limitations
It is important to address three limitations of our study we believe are critical in guiding future taVNS development. Unlike other studies by Frangos and Yakunina, our findings did not reveal any brainstem activation in the regions known to be the vagus entrance into the CNS such as the NTS or LC due to methodological constraints. The human LC is a remarkably small brain region, measuring roughly 1–2 mm at widest in the axial plane [
55]. It is difficult to image such small regions without conducting advanced imaging techniques utilizing rapid, thin slice acquisitions targeting strictly the brainstem. Our scanning sequences were optimized to capture the whole brain hemodynamics at a voxel size of 3 mm
3 which limits the ability to capture not only the structural detail of the NTS and LC, but also its fMRI BOLD response. Future studies should employ specific advanced taVNS/fMRI midbrain and brainstem imaging as well as whole brain scanning.
Our active stimulation site was the left tragus and control region the earlobe. This was based on a series of autonomic studies conducted by our group investigating the effects of tragus stimulation. We cannot determine whether the tragus is the optimal taVNS target as we did not include a cymba conchae target. Both the cymba conche and tragus have been suggested to be biologically active sites and future studies should explore these targets in a head to head fashion to determine optimal stimulation site.
Lastly, our taVNS duty cycle was chosen to be consistent with our prior physiological trials outside of the scanner. The safety profile of stimulation longer than one minute was unknown and we did not want to risk adverse events in the MRI scanner, although upon completing this trial, we believe that stimulation periods longer than 60s would introduce minimal safety risk and would potentially illicit a measurable brainstem BOLD response as demonstrated by Frangos and Yakinuna. There may also be residual cortical brain effects persisting in the 60s inter-stimulation rest blocks that we cannot control for due to scanning procedural constraints. However this type of design was used to increase the power of our effect by increasing the number of stimulation blocks while minimizing scanner drift inherent in long stimulation trials.
Conclusion
These findings demonstrate that taVNS (delivered at 500 μs, 25 Hz) to the left tragus for one minute produces significant cortical effects in the vagal afferent pathway compared to control stimulation of the earlobe. These findings are similar to prior taVNS imaging studies revealing that stimulating the ear at specific regions thought to have ABVN innervations activate afferent vagal networks. Future taVNS/fMRI trials are needed to explore the effects of changes in stimulation parameters on the BOLD signal response.