An Additive Mix? Acute Urinary Retention in a Patient With Benign Prostatic Hyperplasia Treated With Suboxone, Lurasidone, and Trazodone
Abstract
Abstract
Case
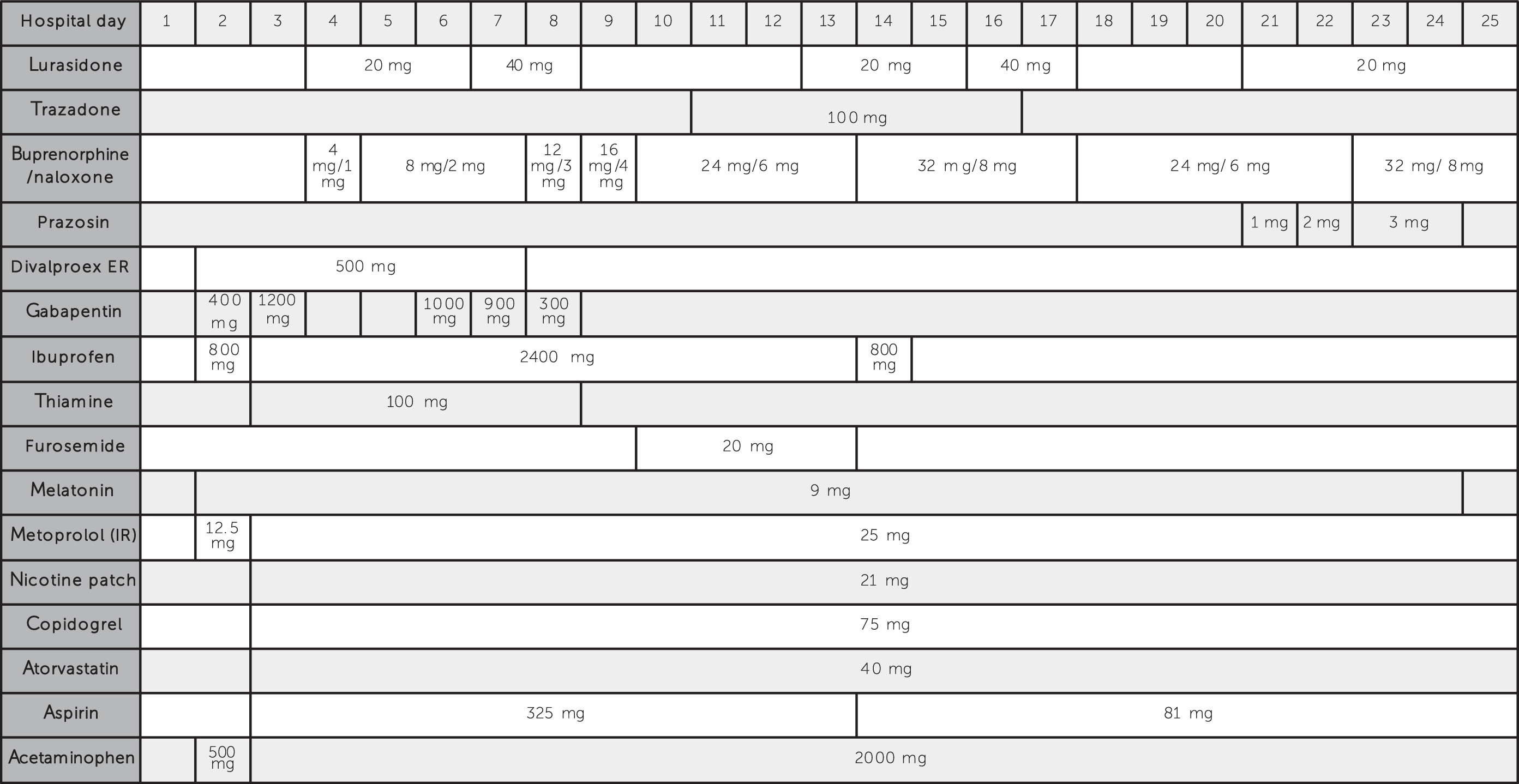
Discussion
| Receptor | Effect | Result |
|---|---|---|
| Central | ||
| D1 | Facilitates storage | Bladder filling |
| D2 | Facilitates micturition | Promotes micturition |
| 5HT2a | Promotes external urethral sphincter contraction through activity at sacral plexus | Bladder filling |
| 5HT2c | Some inhibition of the central micturition reflex and promotion of external urethral sphincter contraction through activity at the sacral plexus | Bladder filling |
| Mu | Indirectly reduces afferent parasympathetic activity from the bladder, resulting in a reduced sensation of bladder fullness; indirectly increases efferent sympathetic activity on the internal urethral sphincter | Bladder filling |
| Peripheral | ||
| Beta-3 | Detrusor relaxation | Promotes micturition |
| Alpha-1 | Internal urethral sphincter contraction | Bladder filling |
| M2 | Detrusor contraction | Detrusor contraction |
| M3 | Main source of detrusor contraction | Promotes micturition |
| Nicotinic | Somatic contraction of external urethral sphincter | Bladder filling |
Lurasidone
Trazodone
Buprenorphine
| Case | Age (Years) | Comorbid Outflow Tract Obstruction | Coadministration of Additional Pharmacologic Agent | Suboxone Maximum Dose | Time to Symptom Development | Treatment | Subsequent Restart and Success |
|---|---|---|---|---|---|---|---|
| Altunsoy et al. (8) | 20 | No | No | 8 mg/2 mg | 2 days | Indwelling catheterization for 3 days + drug discontinuation | No, patient refused |
| Edwards et al. (4) | 49 | No | Unknown | 12 mg/3 mg | 3 days | Indwelling catheterization for 1 week + drug discontinuation | No, patient refused |
| Rai (26) | 58 | BPH | Yes | 12 mg/3 mg | 3 days | Intermittent catheterization + eventual drug discontinuation | Attempts at continuing suboxone failed with recurrence of symptoms |
| Murray (12) | 66 | No | Unknown | 6× 200-μg tablets in 18 hours (buprenorphine alone) | 1 day | Drug withheld | Further use of buprenorphine resulted in recurrent, though self-remitting, anuria |
Pharmacokinetics.
Treatment.
Conclusion
References
Information & Authors
Information
Published In
History
Keywords
Authors
Funding Information
Metrics & Citations
Metrics
Citations
Export Citations
If you have the appropriate software installed, you can download article citation data to the citation manager of your choice. Simply select your manager software from the list below and click Download.
For more information or tips please see 'Downloading to a citation manager' in the Help menu.
View Options
View options
PDF/EPUB
View PDF/EPUBLogin options
Already a subscriber? Access your subscription through your login credentials or your institution for full access to this article.
Personal login Institutional Login Open Athens loginNot a subscriber?
PsychiatryOnline subscription options offer access to the DSM-5-TR® library, books, journals, CME, and patient resources. This all-in-one virtual library provides psychiatrists and mental health professionals with key resources for diagnosis, treatment, research, and professional development.
Need more help? PsychiatryOnline Customer Service may be reached by emailing [email protected] or by calling 800-368-5777 (in the U.S.) or 703-907-7322 (outside the U.S.).

