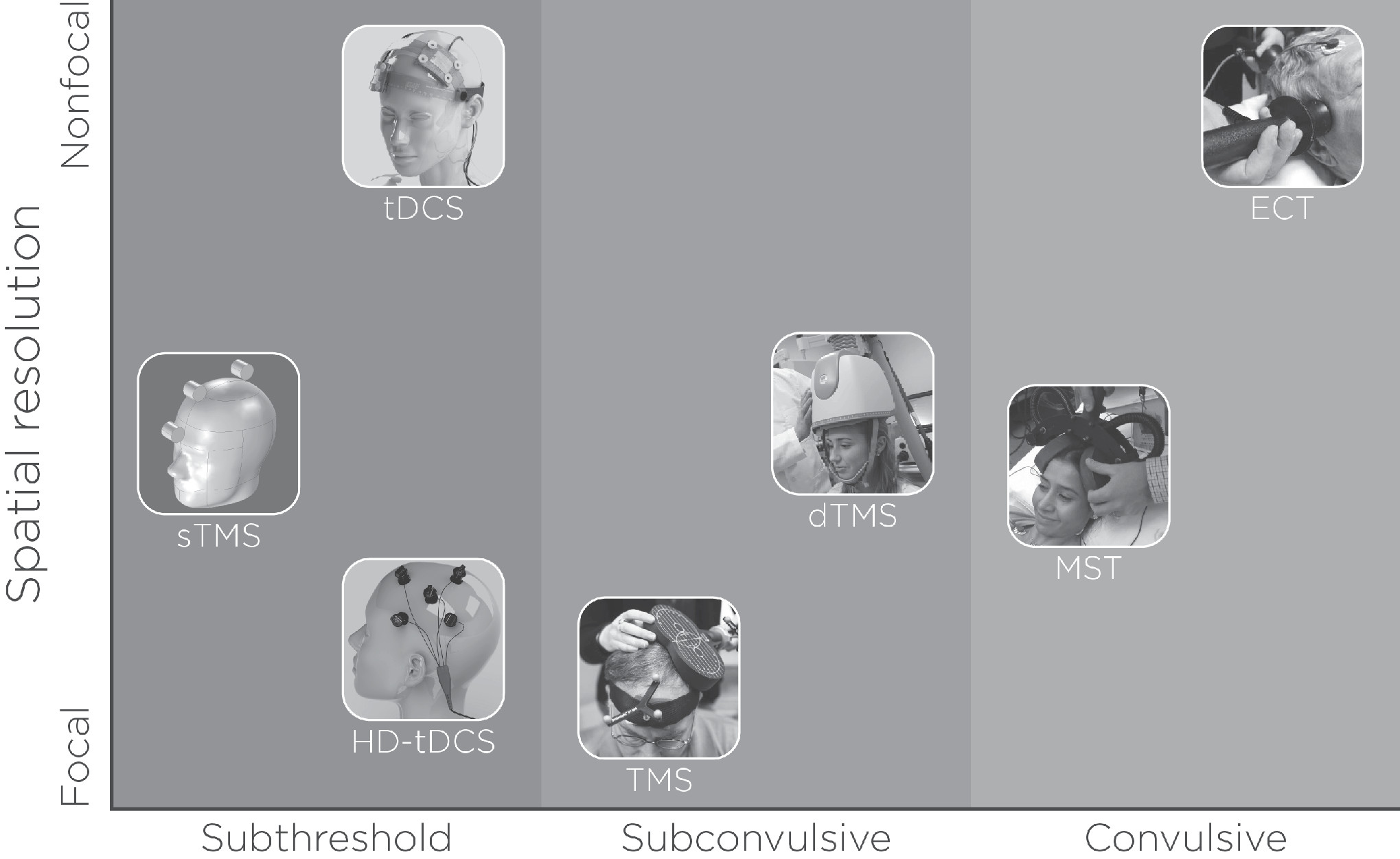
With the prevalence of major depressive disorder and TRD, as well as the associated increased functional impairment, morbidity, and mortality (
12), the past three decades have seen a proliferation of noninvasive neuromodulation therapies (see
Figure 1). In addition to ECT, such therapies include transcranial magnetic stimulation (TMS), magnetic seizure therapy (MST), and transcranial direct current stimulation (tDCS). To date, of these four strategies, only ECT and TMS are cleared for the treatment of depression in the United States by the U.S. Food and Drug Administration (FDA) (
13,
14). MST and tDCS are considered investigational.
The purpose of this critical review article is to synthesize comprehensive information regarding the application of four noninvasive neuromodulation therapies (ECT, TMS, MST, and tDCS) in the treatment of MDD and TRD. Specific information focuses on antidepressant effects, neurocognitive effects, and possible mechanisms of action.
ECT
ECT is a noninvasive neuromodulation therapy that uses electrical stimuli to induce a therapeutic tonic-clonic convulsive seizure. Diagnostic indications for ECT include major depressive disorder or TRD, bipolar disorder, schizophrenia, and schizoaffective disorders (
16). ECT is a first-line treatment when a rapid and definitive response is needed urgently, as in the case of catatonia, risk of harm to self or others, or major depressive disorder with psychotic features. ECT can also be a second-line treatment when medication trials and augmentation strategies have failed to treat an acute mood or psychotic episode.
ECT can be divided into acute, continuation, and maintenance phases of treatment. The acute (also known as
series or
index) phase of ECT consists of approximately 12 sessions administered either twice or three times weekly. Treatment includes individualized end points of remission, clinical plateau, or nonresponse, usually defined by a standardized depression symptom severity measure. Continuation ECT includes the first six months after an ECT acute course and involves either a scheduled taper of treatments from weekly to monthly or symptom-triggered treatments to prolong remission (
17,
18). Maintenance ECT includes regularly scheduled treatments at fixed intervals six months after the ECT acute course to prevent the recurrence of the psychiatric episode (e.g., major depressive disorder) (
19).
ECT has very large effect sizes relative to sham ECT (Cohen’s d=0.91) and pharmacotherapy (Cohen’s d=0.80) (
20), but the ability to predict an individual patient’s response with clinical and demographic factors is modest (
21,
22). Treatment resistance to pharmacotherapy and longer duration of a major depressive episode (longer than two years) are the primary factors predictive of ECT nonresponse. Other factors, such as age, sex, diagnosis (bipolar versus unipolar), and depressive subtype (atypical, melancholic), have not been found to be predictive of clinical outcomes (
21,
22). Although such techniques are not ready for clinical use, recent research has focused on neuroimaging (i.e., magnetic resonance imaging [MRI]) data-driven methods to improve prediction of ECT response (
23–
26) and relapse (
27).
Both the stimulus delivery and the seizure induction seem to work in synergy with respect to clinical response. ECT electrode configuration (d’Elia right unilateral, bifrontal, or bitemporal) dictates the geometry of the induced electric field in the brain (
28). ECT clinicians often start with a focal (right unilateral) and switch to a less focal (bitemporal, bifrontal) electrode configuration in the context of modest improvement midway through the acute ECT course (
29,
30). The ECT pulse train includes pulse width, amplitude, frequency, and train duration.
Although research on quantitative electroencephalography (EEG) analysis has yet to identify the characteristics of the seizure associated with efficacy (
31), most ECT clinicians focus on seizure duration (minimum of 15–20 seconds), with the rationale that shorter seizure duration is reflective of inadequate electric stimulation (
16). Adjustments to total charge to ensure adequate seizure duration include sequential increases in pulse train duration and then frequency (
16,
28). With pulse amplitude and pulse width constant, this method of increasing total charge results in a larger number and faster rate of identical pulses or stimulations. In contrast, increased pulse amplitude would increase the magnitude of the induced electric field, but to date that ECT pulse parameter remains fixed and unadjusted in standard clinical practice (
28).
Pulse width is usually dichotomized into brief (≥0.5 ms) and ultrabrief (<0.5 ms). The ultrabrief pulse width is closer to the chronaxie (stimulus duration required for neuronal firing) but requires increased charge (six times the seizure threshold) with right unilateral electrode placement to maintain clinical efficacy (
29). Collectively, the multiple ECT parameters are used together to produce clinical outcome. For example, bitemporal electrode configuration at brief pulse width and 1.5–2.5 times the seizure threshold, on the one hand, and right-unilateral electrode configuration at brief or ultrabrief pulse width at more than five times the seizure threshold, on the other, produce relatively equivalent clinical outcomes (
32).
The underlying mechanism of action for ECT has yet to be determined. Pretranslational studies have determined that electroconvulsive stimulations result in hippocampal neuroplasticity through increased synaptogenesis, angiogenesis, gliogenesis, and possibly neurogenesis (
33,
34). Consistent with these pretranslational investigations, neuroimaging studies have identified transient increased medial temporal lobe volume during the ECT series (
35,
36). It is interesting that many of these investigations have found larger volumetric increases ipsilateral to the side of direct electrical stimulation (
37–
39). However, there is generally no direct association with decreased depression severity or clinical improvement (
40). ECT’s mechanism of action may also include anticonvulsant mechanisms related to the empiric observation of shortening seizure duration, increased seizure threshold, and increased gamma-Aminobutyric acid (inhibitory neurotransmitter) during an ECT course (
41,
42), but there is no established relationship with changes in anticonvulsant properties and clinical outcomes (
43).
Independent of ECT-associated antidepressant effects, many patients experience transient neurocognitive impairment (
44). The neurocognitive side effects affect all cognitive domains, but anterograde and retrograde memory recall are the domains most affected by the procedure (
45). Immediately after the acute ECT course, cognitive side effects are common (effect sizes between 0.11 and −1.12, depending on the cognitive domain), but patients typically return to the pre-ECT baseline within two to four weeks after the ECT series. The ECT stimulus-delivery parameters differentially affect neurocognitive impairment in the opposite pattern of ECT efficacy, such that focal electrode placement (right unilateral) and ultrabrief pulse widths may be associated with fewer adverse cognitive effects relative to nonfocal electrode placement (bitemporal) and brief pulse widths (
32,
46).
A positron emission tomography investigation demonstrated reduced medial temporal lobe brain metabolism, which was hypothesized to be related to ECT-induced neurocognitive impairment (
47). As such, recent efforts to develop new and more focal ECT stimulation techniques have attempted to avoid stimulation of the medial temporal lobe brain structures (
48). Preclinical investigations demonstrated that hippocampal neuroplasticity and remodeling may adversely affect established memories (
49). However, recent ECT-MRI investigations have neither identified an anatomic location of ECT-mediated neurocognitive impairment nor established a relationship with hippocampal volumetric changes and neurocognitive impairment (
50).
Further work is needed to understand how ECT modulates neurocircuitry to produce beneficial clinical and adverse neurocognitive effects. Clinical research has demonstrated that ECT neurocognitive impairment is unrequired for clinical efficacy (
44). As described above, the role of stimulus parameters represents a trade-off between efficacy and clinical response. In addition, individual variability further confounds the relationship between clinical outcome and stimulus parameters. For example, current ECT practice applies electrical stimuli with fixed pulse amplitude at either 800 or 900 mA, with no clinical or scientific justification (
28). Pulse amplitude and the related electric field are influenced by skin, skull, cerebral spinal fluid, and gray and white matter conductivities, which lead to marked variability despite a constant current at the scalp.
A preclinical investigation with electric field modeling demonstrated that there was more than 50% interindividual variability in the amount of stimulated brain volume with a fixed amplitude with right unilateral electrode configuration (
51). This difference in electric fields could produce markedly different clinical and neurocognitive outcomes despite the application of similar pulse amplitudes at the scalp. To standardize the ECT dose with respect to pulse amplitude, ECT clinicians may use amplitude titration, in addition to other ECT parameters, in the future to eliminate the variability related to anatomic differences to maximize clinical benefits while minimizing neurocognitive effects.
TMS
TMS is a noninvasive neuromodulation technique that uses magnetic induction of an electric field in the brain that is generated from the magnetic field properties of the TMS coil (
52). The electric field produced by TMS can depolarize neurons. Furthermore, TMS pulses can be applied repetitively (i.e., repetitive TMS [rTMS]), which results in excitatory or inhibitory modulation of brain activity in the targeted area and connected brain regions, depending on the stimulation parameters.
TMS has been most widely used as a treatment for major depressive disorder and TRD and was first cleared for the treatment of depression by the U.S. FDA in 2008. In addition, TMS has also been investigated for a variety of neuropsychiatric and neurological conditions, including the treatment of migraine headache (
53), preoperative motor (
54) and language (
55) mapping, treatment for poststroke motor function recovery (
56), auditory hallucinations (
57), and more. Clinical TMS for treatment of depression has generally used high-frequency (≥1 Hz) stimulation administered at 10 Hz to 20 Hz (
58,
59), most commonly targeted to the left dorsolateral prefrontal cortex (L-DLPFC). Less frequently, the right DLPFC has been targeted with low-frequency rTMS (≤1 Hz), a protocol that has been demonstrated through multiple clinical trials to be equivalent to high-frequency TMS in both safety and efficacy (
60).
A relatively new form of TMS-induced neuromodulation, called synchronized TMS, has been explored as a way to deliver TMS stimuli at an individualized alpha frequency determined from real-time integrated EEG recording (
61). This method uses rotating cylindrical magnets to deliver low-energy, sinusoidal-waveform stimulation over a broad area of the cortex. Industry-supported preliminary studies have demonstrated safety and efficacy relative to sham treatment (
62).
To date, three different coil types (focal iron core coil, H1-coil, figure-eight coil) have been cleared by the U.S. FDA. For each magnetic pulse, the peak magnetic field strength generated by the coil is thought to be approximately 1.5 Tesla (
63). Although this magnetic field strength is similar to that of a typical MRI device commonly used in clinical settings, the magnetic field generated by TMS is relatively more focal and brief (
52).
In 2018, the U.S. FDA approved theta-burst stimulation (TBS) for use in the clinical treatment of major depressive disorder. TBS seems to mimic the brain’s endogenous theta rhythms, which is thought to improve induction of synaptic long-term potentiation (
64). TBS has received recent attention, and several clinical trials have emerged that used this method. For example, intermittent TBS, which imparts 600 pulses in three minutes, showed clinical effects that were not inferior to conventional 10-Hz treatment, which lasts approximately 37 minutes (
65). From a clinical practice pragmatic perspective, TBS’s characteristically high-frequency stimulation method may be advantageous because of its shorter per-session treatment duration.
With some exception (
66), an abundance of data pertain to the safety and efficacy of TMS for individuals with major depressive disorder and TRD. Early studies of TMS efficacy found that a two-week rTMS trial (although studies might vary greatly in the parameters used, including stimulation site) was superior to sham or control stimulation (
67). The TMS-associated antidepressant efficacy is comparable to that of pharmacotherapeutic intervention for depression (
68). An industry-sponsored, landmark multisite randomized controlled clinical trial from 23 centers and 301 patients demonstrated that 10-Hz rTMS applied to the L-DLPFC was associated with decreased depression severity (
69). The degree of prior treatment resistance, shorter current episode of depression, and absence of comorbid anxiety disorder predicted better antidepressant outcome with rTMS treatment, which indicates that potential demographic differences may moderate the efficacy of rTMS (
69).
Another multisite, randomized, active sham-controlled study was sponsored by the National Institute of Mental Health. This study investigated daily treatment using the same high-frequency stimulation to the L-DLPFC, provided for up to three weeks (if at three weeks there was no antidepressant benefit, patients were switched to a different TMS condition), among patients with nonpsychotic major depressive disorder or TRD who were free of antidepressant medication. The study demonstrated that active rTMS treatment achieved a remission rate 4.2 times greater than that of sham, with 12 rTMS sessions needed to treat (
70).
Moreover, a study that pooled data from multiple clinical TMS sites with 307 outpatients assessed clinical outcomes, as measured by changes in clinical rating and self-report depression symptom severity scales. Findings showed that TMS produced clinically significant antidepressant effects (
71). rTMS treatments have also been reported to alleviate a broad array of depressive symptom dimensions (e.g., mood, anxiety, somatic), except sleep disturbance, which seems to be resistant to the general clinical benefit provide by TMS (
72,
73). The durability of TMS-associated clinical benefit was demonstrated in a six-month naturalistic follow-up study, such that only approximately 10% of patients seemed to have a relapse of their depressive episode (
74).
Although findings vary across studies and specific cognitive domains, the majority of TMS trials in depression demonstrated improvement or no effect of TMS on cognitive functioning. Of relevance, relative to the temporary adverse cognitive effects reported following ECT (
75–
77), no marked memory inefficiencies or impairments have been found following treatment with a TMS course. A recent review investigated cognitive outcomes across 15 studies among patients with depression who were treated with rTMS (
78). Five of nine studies reported positive effects on executive functioning, one of 12 found positive effects on processing speed, one of five saw improvement in attention, three of 11 found positive effects in verbal memory, and two of seven saw improvement in working memory. It is important to note that a number of the cognitive measures used measured more than one cognitive function; thus, classifying a given measure strictly into a cognitive subdomain could be misleading.
It should be noted that studies of depression measuring cognitive outcomes vary with regard to study methodology, including the presence of a sham condition, clinical trial duration, TMS parameters (e.g., pulse frequency, intensity, train duration), and sample size (with the majority having fewer than 25 participants) (
79). In an earlier review, Guse and colleagues (
80) concluded that high-frequency (10–20-Hz) rTMS applied to the L-DLPFC over 10–15 sessions with a stimulation intensity at 80%−110% of individualized motor threshold was most likely to result in significant cognitive improvement. Collectively, there are discrepant findings regarding the effects of rTMS on cognitive function, and additional research is warranted to confirm whether rTMS modulates cognitive abilities when it is applied for the treatment of major depressive disorder and TRD.
Although direct electrical stimulation of the brain is thought to excite the pyramidal tract axons of neurons directly, TMS is considered to transynaptically excite the pyramidal neurons. The precise underlying mechanism of rTMS-dependent cortical excitability changes, though, remains unknown. However, the long-term effects of rTMS are hypothesized to arise from induction of cortical plasticity, which results from gene induction and protein synthesis (
81).
Depending on the TMS treatment parameters used, TMS can have either an excitatory or an inhibitory effect on local brain function, the latter of which has been used to investigate cerebral function of target regions. The clinical rationale for using rTMS as a treatment tool for major depressive disorder is the notion that rTMS is able to exert a lasting impact on brain function long after discontinuation of the stimulation (
82). As for the underlying biophysical mechanism of high- and low-frequency rTMS, processes similar to long-term potentiation and long-term depression have been suggested as similar molecular mechanisms behind long-term changes that result from rTMS (
83). Currently, though, there is no evidence of synaptic change following rTMS (
84).
Continued work is needed to identify the optimal target, dosage, and duration of TMS treatment for a given individual. Because major depressive disorder is a heterogeneous illness with cognitive, affective, and physiological symptom dimensions, future studies must carefully consider which symptoms will be targeted by TMS and the mechanisms of action that underlie any observed changes. Moreover, it is important to incorporate a personalized medicine approach, investigating which therapies work best and for whom.
For instance, although replication is needed, Drysdale and colleagues, in research with a large multisite sample, suggested that functional connectivity patterns based on functional MRI among patients with TRD could be classified into distinct biotypes and that this classification predicted the patients’ clinical response to rTMS (
85). The DLPFC has been the most frequent site of stimulation used for treating depression with TMS, but other target sites (e.g., right orbitofrontal cortex, dorsomedial prefrontal cortex) may be beneficial for treating particular depressive symptom dimensions. Finally, there is a need to better characterize the network-level changes directly associated with TMS through use of tools such as integrated functional MRI and EEG.
MST
MST is a form of noninvasive neuromodulation convulsive therapy that is currently under investigation. The MST methodology was introduced in 2001 by Lisanby and colleagues in a preclinical study (
86), and the first human studies in TRD were subsequently published (
87–
89). The therapeutic tonic-clonic convulsive seizure in MST is produced by rapidly changing magnetic stimuli that are applied to the brain; the technology is based on rTMS. However, MST differs from TMS in that the magnetic stimuli are administered at much higher stimulation intensities and frequencies (
90). Also, unlike rTMS but similar to ECT, the MST sessions are performed while the patient is under general anesthesia.
The first MST treatments were administered with pulse frequencies of 40–50 Hz (
88,
89,
91), but it was later observed that 100 Hz at maximum stimulator output might more reliably induce seizures (
92). Contradictory results exist, and some studies have suggested that better seizure efficacy could be obtained with lower frequencies of around 20–40 Hz (
28). Further research is needed to confirm the optimal MST pulse frequency, however.
MST can be applied with different electromagnetic coils, such as round, double cone, and cap cone (
93). In recent years, a twin coil that consists of two cone-shaped coils positioned bilaterally has become the most used coil design in application of MST (
94–
96). The stimulation waveform applied in MST is biphasic, which, relative to monophasic pulses, is associated with less coil heating and greater power efficiency (
97).
The general aim of MST is to produce therapeutic seizures and clinical efficacy similar to those of ECT, while avoiding ECT-induced adverse cognitive effects (e.g., anterograde and retrograde amnesia) (
93). MST can produce focal seizures that originate from superficial cortical areas, because magnetic fields can only penetrate approximately 2 cm below the surface of the cortex (
52,
98). The seizure may spread to other brain areas (e.g., DLPFC), similar to that in ECT, and includes both an ictal and a motor phase (
99). However, because MST provides superficial stimulation, unlike ECT, it may avoid stimulating subcortical medial-temporal lobe structures (e.g., hippocampal formation). According to computer modeling studies, MST predominantly stimulates the cortex and approximately 21% of the total brain volume (
100,
101). In addition, because of the use of magnetic fields, MST is different than ECT in that the magnetic stimuli are unaffected by differences in scalp characteristics, skull thickness, or total brain volume (
100).
To date, MST has been successfully applied among patients with TRD (both unipolar depression and bipolar disorder) (
102) and patients with treatment-resistant schizophrenia (
103). Several clinical studies in TRD have demonstrated MST to have antidepressant properties (
88,
89,
104–
106). It is of note that studies that compared and contrasted the efficacy of MST with ECT found no differences between the two therapies in terms of antidepressant clinical outcomes (
95,
104). The response and remission rates in MST have varied around 38%−69% and 15%−46%, respectively (
104–
106), and it has been observed to reduce suicidality (
96).
Regarding cognitive effects, MST has been found to have little to no associated adverse cognitive effects (
93,
102,
107). Instead, preclinical and clinical studies have reported that MST may result in improved cognitive functions in domains such as psychomotor processing speed, visual attention, visuospatial learning and memory, verbal memory, and executive function (
95,
105). The recovery (voluntary movement, respiratory effort, blood pressure, consciousness, and oxygen saturation) and reorientation time associated with MST are also substantially quicker relative to ECT, and patients have been reported to recover within approximately one to two minutes and to become reoriented within approximately three to five minutes after the termination of the seizure (
104). When interpreting these outcomes, one needs to note, however, that the study sample sizes have been small, and studies have not included a sham-controlled condition (e.g., anesthesia only). For example, many studies have incorporated fewer than 20 patients (
93,
99,
102,
107). Thus, further research in larger sample sizes is warranted to confirm and extend the MST safety outcome data.
The exact mechanisms of action of MST remain unknown, but it is assumed that MST may share some of the mechanisms that underlie ECT (
91). For instance, previous positron emission tomography studies have found that MST produced regional glucose metabolism changes in brain areas that have abnormal function in major depressive disorder (
105,
108). Following MST treatments, glucose metabolism increased in the basal ganglia, orbitofrontal cortex, medial frontal cortex, and DLPFC, and glucose metabolism simultaneously decreased in the left striatum (
105,
108).
In most studies, the seizure characteristics measured with ictal EEG have differed between ECT and MST. Relative to ECT seizures, MST seizures commonly have less postictal suppression and smaller EEG amplitude in the ictal phase (
99), and they may have shorter seizure length (
99). However, there is some discrepancy in previous findings, which might in part be explained by the varying stimulation parameters. For example, the applied stimulation frequency in MST has been found to strongly influence the seizure characteristics (
109). The adequacy of an MST seizure is conventionally defined by characteristics that have been noted to result in good clinical outcome in ECT. A recent study, though, challenged this view and found that the MST seizure characteristics might predict clinical outcome differentially than the seizure characteristics in ECT (
109).
In summary, MST has great potential to evolve into a safe and efficacious noninvasive neuromodulation convulsive treatment for major depressive disorder and TRD. Further research is warranted regarding the mechanisms of action underlying MST, optimal stimulus parameters, and associated clinical outcome.
tDCS
tDCS is a noninvasive neuromodulation technique that involves the application of a subthreshold, weak direct electrical current to the brain through electrodes placed on the scalp. Before 2000, the technique was termed
brain polarization and had been investigated in in vivo preclinical models for physiological effects (
110), as well as in early human clinical trials as a treatment for major depressive disorder (
111). Since then, it has been relabeled tDCS and has seen a major resurgence in clinical and research interest following the publication of several seminal studies that demonstrated acute and durable neuromodulatory effects with modern stimulation parameters (
112,
113). This resurgence has been general and spanned the study of neurophysiological mechanisms, neurocognitive effects, and multiple potential therapeutic applications (
114). To date, tDCS remains in development and has no U.S. FDA regulatory clearance for the treatment of any neuropsychiatric disorder.
The current used in tDCS is quite small (i.e., typically 1–2.5 mA), and it flows in a unidirectional manner through the brain, which produces polarity-dependent changes in neuronal excitability (
115). When the technique is used to treat major depressive disorder, the anode (positively charged electrode) generally is placed over the L-DLPFC, with the cathode (negatively charged electrode) placed on the contralateral side of the head. Stimulation is then administered while participants are sitting awake, at rest, for durations of 20 to 30 minutes. tDCS treatments are usually repeated daily over several (e.g., four to six) weeks.
When tDCS is administered this way, there have been mixed results from modern clinical trials (
116). Although several controlled clinical trials have reported significantly greater antidepressant effects with active compared with a sham stimulation (
117–
121), other studies have failed to show superior antidepressant efficacy with active stimulation (
122–
126). In addition, a recent large noninferiority controlled trial among patients with major depressive disorder who were off antidepressant medications found that standard antidepressant medication treatment, relative to tDCS, had superior efficacy (
119). This evidence base therefore suggests that tDCS antidepressant effects are modest when that treatment methodology is used. More recent studies have further investigated the potential for enhanced efficacy when tDCS is administered with newer methodology, such as simultaneously with tasks that concurrently activate stimulated depression-related regions (
127–
129), or when administered with multiple electrodes (referred to as high-definition tDCS) (
130). However, although such newer tDCS methods seem to show promise, additional evidence is warranted from controlled clinical trials to confirm antidepressant efficacy.
The mechanisms underlying tDCS antidepressant effects remain poorly understood. Among healthy participants, tDCS treatment has no mood effects (
131). Among depressed participants, mood effects occur only after several weeks of multiple repeated treatments, analogous to other antidepressant treatments (e.g., rTMS, antidepressant pharmacotherapy). Indeed, tDCS treatment has been shown to cause lasting neuroplastic changes (
132), which is in line with evidence for structural changes with these other treatments (e.g., rTMS (
133), sertraline (
134)). Moreover, tDCS antidepressant effects have been shown to be modulated by the serotonergic system (
121,
135), of which dysfunction is implicated in major depressive disorder and therefore is an important target for antidepressant strategies.
The acute cognitive effects of tDCS have been investigated for their potential role as a mechanism that underlies antidepressant effects. This has followed similar work with pharmacological therapeutic development and the proposed neuropsychological theory of antidepressant effects through modulation of information biases (
136). Consistent with this theory, acute effects on affective biases with tDCS have been demonstrated in several clinical trials among patients with major depressive disorder (
137–
141). To date, however, such effects have been unrelated to antidepressant outcomes (
137). Acute positive effects on other, nonaffective measures have further been shown for processing speed (
120) and visual attention (
141,
142). These heterogeneous findings suggest nonspecific acute neurocognitive effects that involve dysfunctional networks in major depressive disorder, which is consistent with the generalized stimulation effects produced by tDCS (
143).
Another question of clinical relevance is whether a repeated tDCS treatment course for major depressive disorder produces durable cognitive benefit. This was recently addressed in an individual-patient data meta-analysis that involved seven randomized, sham-controlled clinical trials (
144). The results, however, showed that there was no cognitive enhancement following a tDCS course after mood effects were controlled. Similar to tDCS antidepressant effects, further research into alternative treatment methodologies for using tDCS in longer term cognitive enhancement applications is therefore warranted.
Although tDCS continues to remain a potential new treatment for major depressive disorder, current efficacy results indicate a need for further development and refinement in rigorous, controlled, large-scale clinical investigations. tDCS has several advantages relative to other noninvasive neuromodulation antidepressant techniques, including low cost, portability, and enhanced safety (
114,
145). Collectively, these advantages support the translational potential of tDCS.
Promising future lines of research include the development of tDCS as an adjunct to other psychological interventions (e.g., cognitive-behavioral therapy, cognitive training) to further capitalize on its acute neuromodulatory and cognitive effects. Additional research is needed to determine the underlying neuromechanisms that subserve tDCS-induced antidepressant and neurocognitive effects. Emerging evidence has indicated that interindividual differences (i.e., structural and functional) may be promising variables in understanding how tDCS produces beneficial behavioral outcomes (
144,
146).
Conclusions
Noninvasive neuromodulation therapies represent a third wave of antidepressant therapeutic application, after pharmacotherapy and psychotherapy, for the treatment of major depressive disorder and TRD. Those therapies vary in mode of application (electrical or magnetic stimuli), spatial resolution (focal, nonfocal), intensity (subthreshold, subconvulsive, convulsive), antidepressant effects, and neurocognitive effects (see
Figure 1). Considerable research has provided substantial information regarding their antidepressant and neurocognitive effects (see
Table 1).
ECT, as one of the first noninvasive neuromodulation therapies used as an antidepressant strategy, has considerable evidentiary support as being one of the most effective treatments for TRD and major depressive disorder with psychotic features (
4). Research continues to examine how to modify ECT to improve its associated neurocognitive adverse effects (
44). TMS, which was first cleared by the U.S. FDA as an antidepressant strategy in 2008, is useful to treat major depressive disorder and has a benign neurocognitive effect profile. Although its antidepressant effects are mild to moderate, research continues to examine how to maximize antidepressant benefit through new coil types (
62), TMS paradigms (
65), and integration with pharmacotherapeutic (
60) and psychotherapeutic (
147) strategies.
MST and tDCS are relatively newer, noninvasive neuromodulation therapies under development that use magnetic and electric stimuli, respectively, to produce antidepressant effects and minimize neurocognitive effects. Research has found MST to have moderate to high antidepressant benefits for patients with TRD, with no or positive cognitive effects (
88,
95). Research has found tDCS to have modest antidepressant benefits, because of limited understanding of dosimetry (
148), for patients with major depressive disorder, with no or positive cognitive effects (
119,
123).
In conclusion, ECT, TMS, MST, and tDCS are four noninvasive neuromodulation therapies with antidepressant properties that can be beneficial for the treatment of major depressive disorder and TRD. Although the four therapies vary in how they modulate neurocircuitry and their resultant antidepressant and neurocognitive effects, they are nonetheless useful for patients with acute and chronic major depressive disorder and TRD. Continued research is warranted to inform dosimetry, algorithm for administration, and integration among these therapies and with other antidepressant strategies to continue to maximize their safety and antidepressant benefit.