Opioid use disorder and opioid overdose deaths remain an urgent problem despite recent significant efforts in prevention and treatment interventions (
1). Although the opioid crisis has affected people in different age groups, older adults are a potentially vulnerable population that may have been overlooked (
2).
In 2016, an estimated 50 million Americans lived with chronic pain, and roughly 20% of them lived with high-impact chronic pain (defined as pain affecting function and activities of daily living). In addition, the prevalence of chronic pain increases significantly with advancing age (
3). The true incidence of chronic pain in the older adult population is unclear but is estimated to be anywhere from 25% to 85%; one Polish study indicated an estimated prevalence of 40%−50% (
4,
5). The most common areas of pain for older adults are the lumbar region and knees; osteoarthritis is a significant problem among this population. Neuropathic pain and ischemic pain are also important causes of chronic pain (
4,
6). We provide some basic information regarding low back and knee pain in a later section.
Chronic pain (typically defined as pain lasting 12 weeks or longer) in the older adult population is often multifactorial; etiologies include previous traumatic injuries, age-related physiological changes in connective tissue, nervous system degeneration, increasing arthritides from repetitive use and overuse, and consequences of end-stage chronic diseases such as diabetes and various forms of cancer (
7).
The older adult population needs special attention for several reasons. First, older adults are inherently more complicated to treat medically (
8); they often have painful and chronic conditions such as degeneration in bones, joints, and muscles (
9); and they are often prescribed opioid medications for those conditions (
10). Second, elderly patients often take multiple medications, and there may be drug-drug interactions and side effects. Third, compared with younger people, older adults with opioid use disorder appear to be at a higher risk of death, perhaps up to twice as high (
11). Fourth, long-term chronic opioid use among older adults is often rationalized, and opioid use disorder tends to be mis- or underidentified because many elderly people may not need to work or fulfill other obligations outside of their homes, and thus effects of usage may go unrecognized (
12). In addition, some of the symptoms of opioid use disorder may be similar to those of medical conditions common among older adults, as well as to those of depression, delirium, or dementia (
13,
14). Finally, many patients with chronic pain who use prescription opioids have difficulty accepting a diagnosis of opioid use disorder (
15). Many factors may contribute to patients’ reluctance to accept this diagnosis; the most important ones are stigmatization by other people (including health care professionals) (
16) and fear of uncontrolled pain (
17).
The Centers for Disease Control and Prevention’s (CDC’s) analysis of data from the National Health and Nutrition Examination Survey, 2011–2014, found that the mean age of opioid users was 51.8 (95% confidence interval [CI]=49.8–53.7) years, and a larger proportion of opioid users were in the 40–59 and 60-and-older age groups (
18). Another report based on the 2015 National Survey on Drug Use and Health found that the rate of opioid prescribing for older adults is higher than for any other age group (
19). It has been shown that 40%−50% of adults age 50 and older who misuse prescription opioids obtain these medications through physicians (
20) and that older people are less likely to obtain them illicitly (
21).
The data from treatment seekers also show an increase in opioid use disorder among older patients. One study (
2), using data from the Treatment Episode Data Set for Admissions for 2004–2015, found that the proportion of older adults (ages 55 and older) seeking treatment for opioid use disorder rose steadily between 2004 and 2013 (41.2% increase; p-trend=0.046), then increased even more rapidly between 2013 and 2015 (53.5% increase; p-trend=0.009). The proportion of older adults with primary heroin use more than doubled between 2012 and 2015 (p<0.001). Han et al. (
22) analyzed the data of older adults in opioid treatment programs in New York City between 1996 and 2012. They found that, overall, the patients in treatment became older over time, with a large increase in the number of patients ages 60 and older (1.7% in 2006; 13.1% in 2012).
For many patients with chronic pain who may be vulnerable to substance abuse, prescription opioids are often the drugs of choice, at least initially; however, initial abuse of prescription medications may escalate to illicit opioid use (
23,
24). In this context, the distinction between prescription opioid use disorder and illicit (heroin) opioid use disorder may not provide much clinical value.
In this article, we explore the overlap of chronic pain and opioid use disorder in the population of older adults.
Assessment and Management of Chronic Pain Among Older Adults
Chronic pain is difficult to accurately diagnose and treat because of its complex and multifactorial nature (
25). A multimodal approach involving both pharmacological and nonpharmacological methods is required to produce the best improvements in pain and function. Although certain general principles should be followed in the treatment of all patients, pain management plans are more likely to be successful when individualized and when patients are actively engaged in multiple modalities related to pain care (
26).
Initial steps in the workup for chronic pain should include a focused history and physical examination, with a focus on musculoskeletal and neurological exams. Clinicians should emphasize the effects of musculoskeletal factors on pain and any associated radiation of the pain in conjunction with neurological symptoms. Assessment of older adults must weigh the impact on the patient’s ability to perform activities of daily living, how the patient’s independence is ultimately affected by pain, and whether a caregiver is required. To determine whether patient history is accurate, history taking must also account for cognitive impairment (including dementia), language barriers, and other communication barriers; unreliable histories should be supplemented by observers or caregivers (
27). Imaging may be useful, but MRI studies with healthy volunteers have demonstrated pathological imaging findings (such as intervertebral disc bulges) in the absence of pain; this may limit the significance of positive imaging findings, rendering a physical exam particularly valuable (
28).
An important point is that the use of opioids (like all drugs of abuse) results in significant dopamine release into the nucleus accumbens, resulting in euphoria. Coupled with the fact that more than half of the nation’s opioids are likely prescribed to those with mental disorders (
29), this has numerous implications, including the possibility that some patients may use opioids in the attempt to relieve psychological suffering either in addition to or in lieu of physical pain.
Low-Back Pain
Generally, low-back pain is divided into axial and radicular components. Axial back pain is the more common of the two, and most patients with acute axial low-back pain will recover within 6 weeks. Axial low-back pain typically worsens with certain activities or positions and is usually relieved with rest. Nonpharmacological therapies such as active exercise and physical therapy, spinal manipulation, and application of heat or ice are mainstays of treatment and have been shown to be effective (
30). One meta-analysis showed little long-term benefit of cognitive-behavioral therapy (CBT) for low-back pain, but mindfulness has demonstrated moderate effectiveness (
30,
31). In terms of medications, skeletal muscle relaxants, acetaminophen, and nonsteroidal anti-inflammatory drugs (NSAIDs) have demonstrated efficacy in the acute phase, and tricyclic antidepressants have shown some moderate benefit in the chronic phase. Topical treatments such as lidocaine patches and ointments as well as NSAID gels or ointments are often beneficial as well and typically have a better adverse effect profile (
32). Lumbosacral orthotic braces have also been used with some benefit, but evidence for their efficacy is mixed. Patients may also benefit from certain interventional modalities such as direct intra-articular injection of these joints or radiofrequency ablation of the sensory nerves that supply them. Surgery is typically not helpful for pain that is solely axial and is rarely recommended for this indication (
33).
Radicular back pain, or pain associated with a particular nerve root, is also known as sciatica. Sciatica typically occurs secondary to nerve root compression or inflammation and can also be associated with lumbar spinal stenosis. Radicular pain can be associated with weakness and loss of sensation as well. Patients with radicular back pain typically have greater pain in the legs than in the back; neuropathic pain (described as numb, tingling, pins and needles, and often electric, burning, or shooting) is typical (
34).
Radicular pain is often more difficult to treat, although some benefit has been shown with off-label use of anticonvulsants (
34). Patients with radicular pain are more likely to benefit from physical therapy as well as from interventional modalities, including but not limited to epidural steroid injections, spinal cord stimulation, and minimally invasive lumbar discectomy treatments. If radicular symptoms do not improve with nonsurgical treatment, spine surgery can be considered; that said, if neither nerve root compression nor spinal stenosis is clearly documented on an imaging study, surgical intervention is unlikely to be helpful with symptoms (
35).
Knee Pain
Knee pain is extremely common among older adults. The most common causes of knee pain are typically related to aging, prior injury, or repetitive use of the knee. Osteoarthritis is the most common disease of the knee; pain is due to degenerating articular cartilage within the joint (
36).
Knee pain typically responds well to physical therapy and to anti-inflammatory medications, whether systemic or topical. Interventional procedures such as corticosteroid injections or hyaluronic acid injections are usually beneficial and often give patients relief for several months at a time. Surgical intervention is typically reserved for severe or refractory cases (
37).
Challenges With Pain Management Among Older Adults
Issues related to nonpharmacological treatments include decreased mobility or challenges associated with transportation, which can preclude participation in physical and occupational therapies as well as regular exercise programs; this lack of participation may promote lack of movement as a whole, which can eventually lead to disuse muscle atrophy and gradual degeneration of important anatomical structures such as intervertebral discs, muscles, and ligaments. In addition, some of the aforementioned more invasive care options for pain such as interventional pain treatments and joint replacement surgeries can be precluded by anticoagulation, osteoporosis, cardiac disease, and dementia, all of which are more common among older adults. Decreases in daily functional status and the ability to perform previously enjoyable activities or interact meaningfully with friends and family as a result of pain can also induce or worsen mood disorders among this population (
38).
As a result of age-related physiological changes, pharmacokinetics and pharmacodynamics can be significantly altered among older adults. Pharmacokinetically, distribution is reduced secondary to decreased protein binding and decreased total body water, and drug metabolism is reduced because of declines in hepatic and renal function. This functional decline is expected, although lab values may be within the normal limits. These declines in hepatic and renal function are thought to be due to decreased liver volume and decreased first-pass hepatic metabolism and to decreased glomerular filtration rate and renal blood flow, respectively. These changes can particularly result in longer half-lives for medications with high hepatic extraction ratios such as certain opioids (morphine, meperidine, methadone, levorphanol) and NSAIDs such as celecoxib, diflunisal, naproxen, oxaprozin, piroxicam, salsalate, and sulindac. Pharmacodynamically, opioid-receptor sensitivity increases with the associated physiological decline in mu-opioid receptor density associated with aging; this population may have more significant clinical effects with small doses of opioids (
39).
These declines in medication clearance make use of high-risk medications (benzodiazepines and other sedatives) even more concerning, particularly if paired with other sedating pain medications. Among older adults, falls are associated with significant morbidity and mortality, and the reduction in metabolism can significantly increase the possibility of one of these catastrophic events.
The 2019 update to the American Geriatrics Society Beers Criteria maintains the recommendation to avoid anticholinergic agents, including antihistamines and tricyclic antidepressants, for this population. Opioids and benzodiazepines, anticonvulsants, and other sedating antidepressants and so-called “Z-drugs” such as zaleplon, eszopiclone, and zolpidem should generally be avoided alone or in combination when possible (
26).
Prevention of Opioid Use Disorders Among Older Adults
The overall approach to prevent opioid use disorder among older adults is similar to that for younger adults. When considering prescription opioid medications for acute pain for older adults, it is important to use the lowest effective dose of the least potent immediate-release opioid for a duration of ≤3 days and rarely >7 days (
10,
12,
40,
41).
The value of opioids in treating chronic nonmalignant pain remains controversial because there are no long-term studies demonstrating prolonged and sustained improvement in function with chronic opioid therapy. At least one randomized controlled trial has demonstrated no significant difference in improvement in pain-related function between opioids and NSAIDs for low-back, hip, and knee pain, which are some of the most common pain conditions among older adults (
42).
Although the adverse effects of opioids are well known, particularly in the context of the ongoing opioid crisis, patient selection for opioids continues to be a challenge. Some patients continue to derive benefit in terms of pain-related function with minimal or no adverse effects, and others experience substantial issues related either to adverse effects or to development of addiction. Most prescription opioid users are elderly, and several of the signs of opioid use disorder may be masked by reduced social obligations, as previously indicated. Many practitioners use validated screening tools such as the Opioid Risk Tool to document risk factors before initiating therapy and the Current Opioid Misuse Measure to evaluate ongoing risk of continuing therapy; validated tools for functional assessment, such as the Oswestry Disability Index, are also useful for assessing improvements in function with medications.
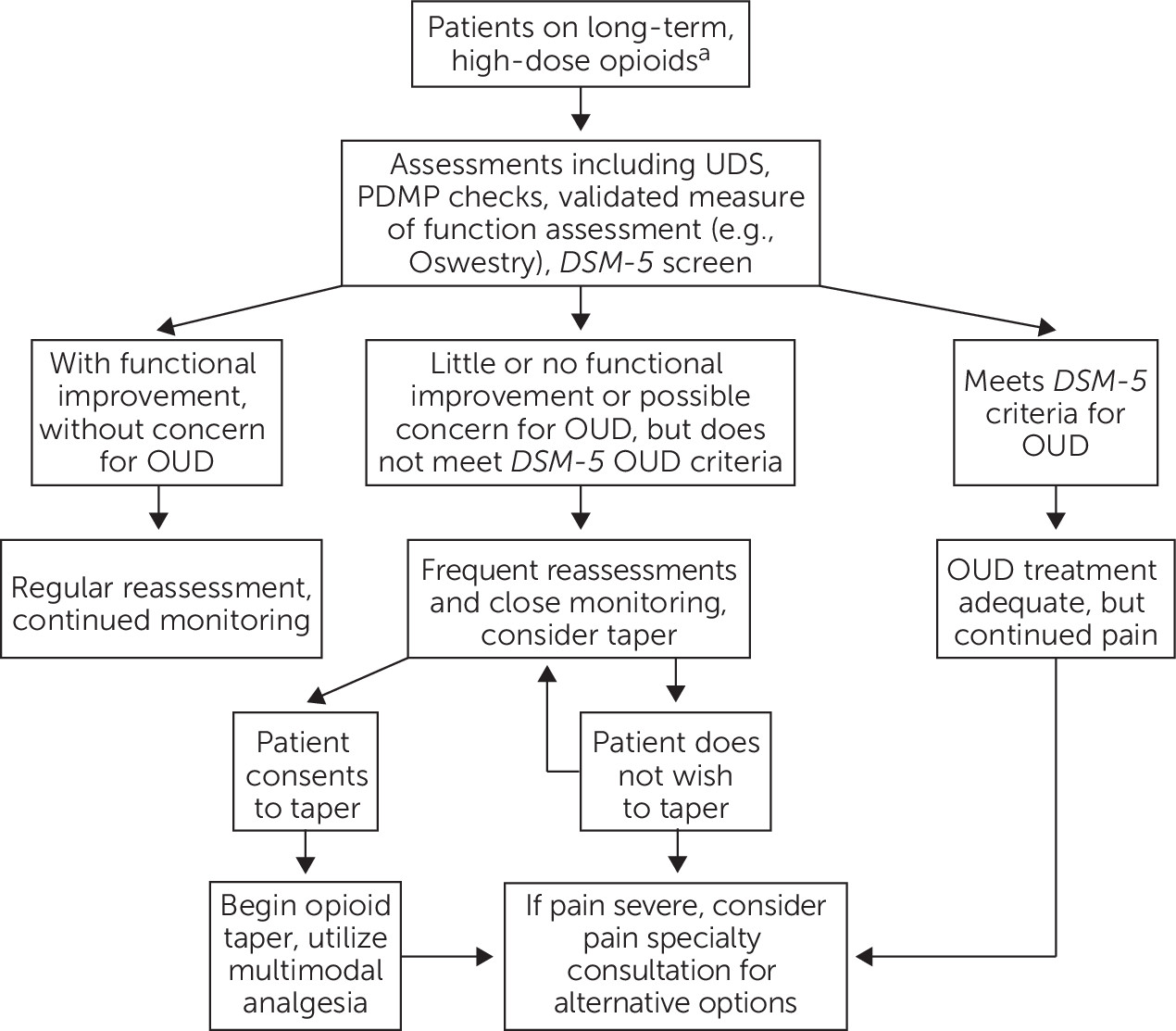
For most patients with chronic noncancer pain, long-term opioids should be avoided. A careful trial of opioids may be considered for older adults with severe pain that is not responsive to nonopioid therapy, but clinicians need to be particularly cautious with patients with a history of substance use disorder or active mental health disorders. Obtaining a second opinion can sometimes be helpful; in addition, the initial and maintenance doses of opioids for older adults should ideally be lower than for younger adults, and medications should be discontinued if function does not improve or if adverse effects arise (
10,
43) (
Figure 1).
For all of these reasons, only the lowest effective opioid dose should be used. In addition, tapering or discontinuing the opioid and other medications should be considered in certain circumstances (
10,
43,
44). However, some recent studies have suggested that nonconsensual discontinuation or tapering may be associated with adverse outcomes, such as increased emergency room visits, hospitalization, and overdose (
45).
To minimize the risk of overdose, educating patients and their caregivers regarding medication safety is essential. According to the 2016 CDC guidelines, clinicians should strongly consider dispensing naloxone kits to all patients (or to their family members) who use opioids regularly for any reason (
10).
Screening and Assessment
For older adults who use opioids regularly, it is important to screen for and identify an opioid use disorder. Early identification and diagnosis rely on comprehensive assessment, including both medical and psychiatric assessments (see
Figure 1). Assessments need to include pain and functional assessment, along with substance use assessment including urine toxicological testing and use of validated, structured instruments. In addition, clinicians should regularly review patients’ medications, including searching the online prescription drug monitoring programs (
46). Reassessment is essential and should be conducted periodically throughout long-term care (
47,
48).
The clinical features of chronic pain and opioid use disorder have multiple points of interface (
49), and teasing these out from each other can be challenging. In this context, it is useful to describe the differences among physiological opioid dependence, complex persistent opioid dependence, and opioid use disorder.
Physiological opioid dependence is strictly a pharmacological result of opioid use, whether prescribed or illicit. Opioid dependence is defined by the presence of withdrawal symptoms in the absence of opioid use or use in reduced amounts. In
DSM-5, the tolerance and withdrawal criteria are not considered to be met for those individuals taking opioids solely under appropriate medical supervision (
50). Complex persistent opioid dependence currently does not carry an
ICD-10 code (
51,
52). It describes a constellation of symptoms that include poor pain control; declining function; psychiatric instability, medical instability, or both; and aberrant behaviors when opioid tapering is attempted (
52). Opioid use disorder, per the
DSM-5, is defined by maladaptive, risky, and pathological behaviors associated with opioid use. These problematic behaviors specifically define opioid use disorder and are not part of the criteria associated with either physiological opioid dependence or complex persistent opioid dependence (
50).
Although fully addressing the characteristics of complex persistent opioid dependence is outside the scope of this article, it may provide some utility to discuss therapeutic options. If a chronic pain patient is using opioids long term without significant clinical benefit (i.e., improvement in function), one can reasonably consider tapering the medication. That said, if function decreases substantially and aberrant behaviors begin to surface, one should consider a diagnosis of either complex persistent opioid dependence or opioid use disorder. Buprenorphine (and to a lesser extent, methadone) have demonstrated some clinical efficacy in complex persistent opioid dependence, as has CBT for chronic pain (
52).
Screening for opioid use disorder in the population with chronic pain requires both time and expertise. One significant challenge is that patients who desire more medication may have tolerance, previous undertreatment of pain, or worsening of the underlying condition causing pain (e.g., cancer); alternatively, it may indicate the manifestation of a substance use disorder. These presentations can be subtle and difficult to differentiate. In assessing patients with such conditions, we have used an approach in which they are assessed by both a pain specialist and an addiction psychiatrist in a pain clinic setting, with pain evaluation as the primary focus; in addition, a full psychiatric assessment is also conducted with a focus on an opioid use disorder diagnosis.
On the basis of both the literature (
53) and clinical experience, some behaviors are more suggestive of prescription opioid use disorder: deterioration in function (work, social); illegal activities (selling medication, forging prescriptions, buying from nonmedical sources); altered route of administration (injecting, snorting); repeated episodes of lost or stolen prescriptions; resistance to change therapy despite negative outcomes or lack of efficacy for pain; refusal to comply with toxicology testing; concurrent, active abuse of alcohol or other illicit drugs; and use of multiple physicians or pharmacies to obtain prescriptions.
The following behaviors, however, are often less suggestive of prescription opioid use disorder: complaints for more medication; medication hoarding; requesting specific pain medications; transparently acquiring similar medications from other providers; occasional unsanctioned dose escalation (openly); and nonadherence to other recommendations for pain therapy.
Treatment of Opioid Use Disorder Among Older Adults
Overview and Psychosocial Interventions
As with younger patients with opioid use disorder, treatment for older patients with opioid use disorder involves comprehensive, interdisciplinary efforts, and this approach is further emphasized for elderly patients with chronic pain and opioid use disorder. A collaborative approach, including substance abuse treatment and pain management involving medical and behavioral health care professionals, tends to have a better outcome (
54,
55). The important treatment components often include psychosocial interventions and medications for opioid use disorder, formerly known as medication-assisted treatment.
It is important to tailor the treatment plan on the basis of each patient’s individual needs; older patients often face unique issues with regard to substance use, and they often need both individual and group treatments (
56). Depending on the demographics and backgrounds of other participants, older adults not infrequently feel uncomfortable or shame when discussing their problems with younger persons in group settings.
One important treatment component is to help patients process and accept the new diagnosis and to motivate them to seek treatment. Motivational interviewing techniques have been shown to be helpful in this regard. CBT and acceptance and commitment therapy may help patients accept their diagnosis and commit to living a values-based life (
57). Another study showed that mindfulness-oriented recovery enhancement was effective with patients with chronic pain and risk of opioid misuse (
58).
Current evidence does not support abstinence-based therapy for opioid use disorder. Detoxification by itself is usually not sufficient to produce long-term recovery (
59,
60), and it may actually increase patients’ risk of overdose. A well-known phenomenon is that patients often lose their tolerance to opioids because of medical detoxification or a period of forced abstinence (e.g., during incarceration) (
59,
61–
63).
The current scientific literature overwhelmingly supports medications playing a central role in treating opioid use disorder (
60,
64–
66), and the most commonly used medications are buprenorphine (with or without naloxone), methadone, and naltrexone. Among these, buprenorphine-naloxone is widely considered the first-line therapy for opioid use disorder among older adults.
Data from the Prescription Opioid Addiction Treatment Study (
67) showed that older patients had better outcomes when treated with a medication for opioid use disorder, especially those who had never used heroin or had initially used opioids for medical reasons (pain). This study also found a few indicators for poorer outcomes: a history of heroin use, having more severe problems, and using opioid medications via a route or in a way in which it was not intended (e.g., snorting, crushing, chewing). Somewhat unexpectedly, patients with both opioid use disorder and major depressive disorder had nearly twice the odds of achieving a successful outcome.
In treating patients with opioid use disorder, prescribers are encouraged to prescribe the overdose-reversing medication naloxone in the form of rescue kits to patients and their caregivers and to educate the relevant parties in its use (
64,
66).
Medications for Opioid Use Disorder
Current evidence-based guidelines recommend combining medications for opioid use disorder with individual and group therapy or other supportive services (
10,
66,
68). The data show that medications can lead to clinical improvements for patients and improvements in their quality of life and health outcomes with increased length of time on medications for opioid use disorder (
64). Of note, evidence focused on geriatric opioid use disorder is limited. Much of the current data on geriatric opioid use disorder comes from larger populations that included older adults in their demographic (
69).
In 2011, Rosen et al. (
70) conducted a comprehensive literature review of studies of older adults (age 50 years or older) who use heroin. They found that all the studies used convenience samples, and seven of the nine eligible studies (77.8%) were entirely drawn from substance abuse treatment programs (primarily methadone maintenance programs). They found that older heroin users often felt marginalized, and the experience often had a negative impact on their treatment seeking, treatment outcome, and treatment retention. They also found that small sample sizes and inconsistent findings on methadone treatment for heroin abuse among older adults limit the data available on this topic.
Buprenorphine.
Buprenorphine is a mu-opioid receptor partial agonist and kappa-opioid receptor antagonist. The partial mu agonist effect decreases the risk of respiratory depression except when it is used with other central nervous system depressants (
71). Because of its ceiling effect and better safety profiles, lower risk of QTc prolongation, and fewer clinically significant drug interactions, buprenorphine is a safer option than other choices of medication for opioid use disorder (
72,
73).
Multiple buprenorphine formulations are approved for both pain management and treatment of opioid use disorder. Those available for treatment of opioid use disorder include sublingual or buccal buprenorphine (2 mg or 8 mg) tablets or films, with or without naloxone; the products without naloxone are available for patients who cannot tolerate the buprenorphine-naloxone combination. It is generally agreed that buprenorphine-naloxone or buprenorphine maintenance should be considered as a first-line treatment for older adults with opioid use disorder (
47,
48,
66,
68). Because older adults have lower metabolism, some researchers have suggested that they may need a lower start dose or a longer time to dose escalation (
74).
Another opioid use disorder formulation that is available is a monthly injection of buprenorphine (100–300 mg; Sublocade); a recent study (
75) found that the monthly injectable treatment led to positive patient-centered outcomes, high treatment satisfaction, and personal recovery.
Methadone.
Methadone is a long-acting mu-receptor agonist that has been used for pain and opioid use disorder. Methadone used for the treatment of opioid use disorder can be provided only through a federally licensed opioid treatment program. Because of its long half-life, methadone is administered once a day for opioid use disorder. During the induction phase, the initial dose should not exceed 30 mg, and subsequent daily doses can range from 60 to 120 mg.
As a result of its metabolism through the cytochrome P450 enzyme systems (
73), drug-drug interactions may occur. Studies have also found that the risk of overdose during the first 4 weeks of treatment can be a clinical concern (
72). Recently, a study from the United Kingdom (
76) found that illicit opioid users’ risk of methadone-specific death increases with age. Specifically, relative to ages 25–34 years, the pooled hazard ratio for methadone-specific deaths was 3.75 at age 45 and older (95% CI=2.99–4.70). In addition, due to notable QTc prolongation, a baseline electrocardiogram (ECG), and periodic ECGs every 6–12 months, are recommended (
77).
Naltrexone.
Naltrexone is a mu-receptor antagonist that was approved for the prevention of opioid use disorder relapse in 2010 (
47). Naltrexone can be initiated only after the individual has detoxified from opioids and been opioid free for at least 7 to 10 days. Naltrexone is not a controlled substance, and it can be prescribed by any licensed practitioner.
Naltrexone has two formulations: oral and intramuscular depot injectable naltrexone (extended release). The use of oral naltrexone for the treatment of opioid use disorder is limited because of its lower rate of patient acceptance, the need to go through opioid withdrawal and maintain this state for an adequate period, and high rate of medication nonadherence (
78). In fact, one meta-analysis showed that oral naltrexone was not superior to placebo or to no medication in treatment retention or illicit drug use (
79). The US Department of Veterans Affairs/Department of Defense practice guidelines (
68) do not recommend its use for opioid use disorder.
However, multiple studies have shown that extended-release naltrexone (XR-NTX) is more effective than placebo or no medication in reducing relapse on opioid use (
80,
81). A few studies have compared XR-NTX with buprenorphine. Current evidence suggests that it is more difficult to initiate XR-NTX treatment because it often requires medical management of opioid withdrawal first; however, once initiated, both medications were equally safe and effective (
82,
83). One of these studies showed that among participants who were randomly assigned, patients in the XR-NTX group had a higher rate of relapse (65% in XR-NTX group vs. 57% in buprenorphine-naloxone group) (
82).
Currently, most guidelines do not recommend naltrexone as a first-line treatment for older adults with opioid use disorder. It can be offered for those for whom buprenorphine or methadone might be contraindicated, who decline agonist therapy, who are without access to agonist treatment (such as those in prisons or certain professions), or who have established abstinence for a sufficient period of time (
48,
66,
80,
84).
Treating Chronic Pain in the Setting and Context of Opioid Use Disorder
Treating chronic pain in the context of opioid use disorder is a challenging balance of adequate pain management and attention to safety in this particularly vulnerable population. Although obvious efficacy limitations (based on extent of pathology and individual pain tolerance) exist, nonpharmacological methods are preferred as a first-line treatment.
Medication treatment for opioid use disorder should be recommended if appropriate. Optimizing the dosage of methadone or buprenorphine-naloxone may provide pain relief. Buprenorphine carries a Food and Drug Administration indication for pain in its microgram formulations (transdermal patch and buccal), and the milligram sublingual formulations are sometimes used off-label for pain. For treatment of chronic pain, methadone is typically used in much lower doses (e.g., 5–30 mg daily, usually split) than for opioid use disorder. Prescribing methadone to older adults requires caution because of its black box warning for QT prolongation and its potential for significantly increased duration of action secondary to long half-life, particularly in the context of decreased metabolism. One study compared the analgesic efficacy of methadone and buprenorphine in the population with opioid use disorder and found that fewer than half (48.1%) of the patients remained in the study at the end of 6 months; they reported a 12.75% improvement in pain scores but no significant improvement in overall pain or function at this same time interval with either medication. However, it is notable that five patients in the buprenorphine group reported illicit drug use, whereas no patients in the methadone group did so (
85).
For a patient with diagnosed opioid use disorder and chronic pain, full opioid agonists should ideally be avoided unless for acute pain control (such as surgery, injuries, etc.), including for patients who are managed on opioid agonist therapy for opioid use disorder. For chronic pain, use of adjunctive nonopioid medications (systemic and topical) is typically helpful; if pain continues to be a substantial issue in terms of overall functionality in spite of multiple trials of these treatments and multimodal therapy, clinicians should consider specialty pain management or surgical consultation, depending on the nature of the pain generator.
As a mu-receptor antagonist, naltrexone does not carry substantial analgesic benefit for most patients. It has interestingly been shown in very low doses (e.g., 1–5 mg) to provide some improvement in pain and function to those with centralized pain states such as fibromyalgia (
86).
Last, it is worth mentioning that a recent systematic review of 12 studies of pain among patients with opioid use disorder found some preliminary evidence for analgesic and antihyperalgesic effects of gabapentin, gamma-aminobutyric acid agonists, and
N-methyl-D-aspartate antagonists for pain (
87).
Conclusions
The interface between chronic pain and opioid use disorder in the older adult population needs special attention because of unique factors related to physiological changes and altered metabolism. Routine screening and awareness are key to identifying opioid use disorder. Complete assessments need to encompass pain assessment (including functional status and substance use assessments, including the use of urine toxicological testing) and structured instruments. Treatment for older patients with opioid use disorder involves comprehensive, interdisciplinary efforts; this approach is even more critical with elderly patients with chronic pain and opioid use disorder. A collaborative approach including substance abuse treatment and pain management (including pain subspecialty care) is recommended. Although medications for opioid use disorder are essential in most cases, psychosocial interventions may also play an important role in particular situations.
Acknowledgments
The authors thank Dr. Charlotte Van Hale, Dr. Santosh Patel, and Dr. Christopher Mora of the Department of Psychiatry and Behavioral Science at Emory University, who provided thoughtful and invaluable comments on drafts of this article.
The authors report no financial relationships with commercial interests.